加比亚抗噬菌体超分子组装的分子基础
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
作为原核生物中最普遍的抗噬菌体防御系统之一,Gabija 由 Gabija 蛋白 A(GajA)和 Gabija 蛋白 B(GajB)组成。Gabija系统的组装和功能仍不清楚。在这里,我们展示了蜡样芽孢杆菌 GajA 和 GajAB 复合物的冷冻电镜结构,分别揭示了四聚体和八聚体的组装。在复合物的中心,GajA组装成一个四聚体,它在复合物的相对两侧招募了两组GajB二聚体,从而形成了一个4:4的GajAB超分子复合物,用于抗蚜虫防御。进一步的生化分析表明,单靠 GajA 就足以切割双链 DNA 和质粒 DNA,而 ATP 可以抑制这种切割。意想不到的是,GajAB 对质粒 DNA 的活性增强了,这表明 GajB 在底物选择方面发挥了作用。总之,我们的研究为了解 GajAB 复合物的抗噬菌体免疫防御功能提供了一个框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
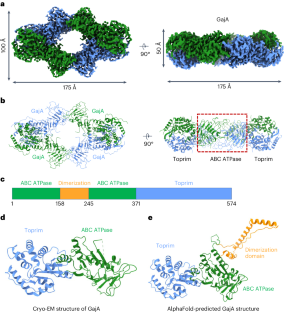
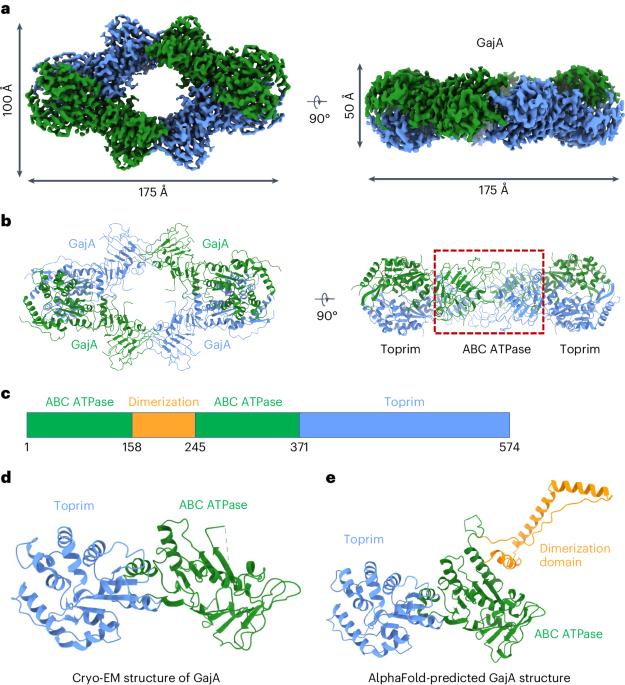
Molecular basis of Gabija anti-phage supramolecular assemblies
As one of the most prevalent anti-phage defense systems in prokaryotes, Gabija consists of a Gabija protein A (GajA) and a Gabija protein B (GajB). The assembly and function of the Gabija system remain unclear. Here we present cryo-EM structures of Bacillus cereus GajA and GajAB complex, revealing tetrameric and octameric assemblies, respectively. In the center of the complex, GajA assembles into a tetramer, which recruits two sets of GajB dimer at opposite sides of the complex, resulting in a 4:4 GajAB supramolecular complex for anti-phage defense. Further biochemical analysis showed that GajA alone is sufficient to cut double-stranded DNA and plasmid DNA, which can be inhibited by ATP. Unexpectedly, the GajAB displays enhanced activity for plasmid DNA, suggesting a role of substrate selection by GajB. Together, our study defines a framework for understanding anti-phage immune defense by the GajAB complex. The Gabija system constitutes one of the most prevalent anti-phage defense systems and is composed of GajA and GajB. Here, using cryo-EM and biochemistry, the authors show that GajA and GajB form a supramolecular complex with a stoichiometry of 4:4 to promote anti-phage defense.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


