卒中量分配模型使可穿戴传感器用于血管年龄和心血管疾病评估
IF 12.3
1区 材料科学
Q1 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
摘要
利用消费类电子产品对心血管状况进行频繁而不显眼的监测是人们普遍追求的目标,因为它是预防和控制心血管疾病(CVDs)--全球主要死亡原因--的最经济、最有效的方法。然而,由于缺乏将测量信号与心血管状况联系起来的生理模型,目前大多数可穿戴设备和柔性设备只能支持心率和血氧水平等一两种生命体征的测量。在此,我们报告了一种卒中量分配(SVA)模型,该模型可量化动脉的缓冲功能,并赋予几乎所有现有心脏传感器新的功能,包括动脉僵化评估、动态血压跟踪和心血管疾病相关心脏损伤分类。为了验证 SVA 模型,我们进行了大规模临床数据测试,其中包括来自 6 家医院/研究机构的混合数据集(9 个开放数据库和 4 个自建数据库,共计 878 名受试者)以及不同的测量方法。结果表明,基于 SVA 的参数与动脉僵化和血压的黄金标准测量值具有良好的相关性,在检测心血管系统异常方面优于常用的单独生命体征(如血压)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
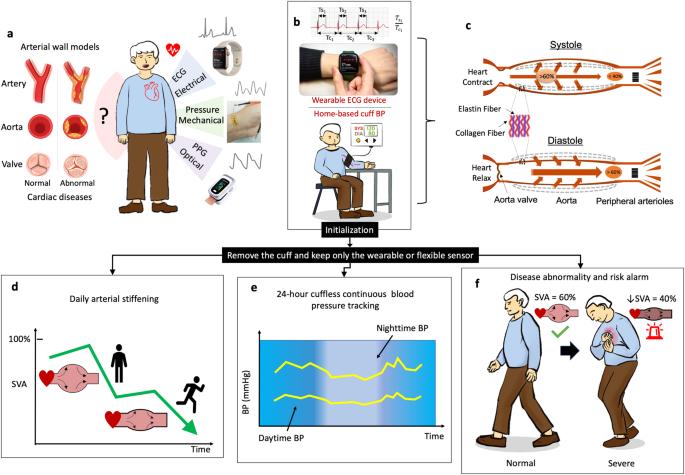
Stroke-volume-allocation model enabling wearable sensors for vascular age and cardiovascular disease assessment
Frequent and unobtrusive monitoring of cardiovascular conditions with consumer electronics is a widely pursued goal, since it provides the most economic and effective way of preventing and managing cardiovascular diseases (CVDs) ─ the leading causes of death worldwide. However, most current wearable and flexible devices can only support the measurement of one or two types of vital signs, such as heart rate and blood oxygen level, due to the lack of physiological models to link the measured signals to cardiovascular conditions. Here, we report a stroke-volume allocation (SVA) model to quantify the cushioning function of arteries and empower nearly all existing cardiac sensors with new functions, including arterial stiffness evaluation, dynamic blood pressure tracking and classification of CVD-related heart damage. Large-scale clinical data testing involving a hybrid dataset taken from 6 hospitals/research institutes (9 open databases and 4 self-built databases from 878 subjects in total) and diverse measurement approaches was carried out to validate the SVA model. The results show that the SVA-based parameters correlate well with the gold-standard measurements in arterial stiffness and blood pressure and outperform the commonly used vital sign (e.g., blood pressure) alone in detecting abnormalities in cardiovascular systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

npj Flexible Electronics
Multiple-
CiteScore
17.10
自引率
4.80%
发文量
91
审稿时长
6 weeks
期刊介绍:
npj Flexible Electronics is an online-only and open access journal, which publishes high-quality papers related to flexible electronic systems, including plastic electronics and emerging materials, new device design and fabrication technologies, and applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


