从软珊瑚 Capnella imbricata 中分离出的新类固醇 Capnesterones A 和 B
IF 1.3
4区 生物学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
研究人员从台湾东南海域的软珊瑚 Capnella imbricata 中分离出了两种新的非烷基固醇类化合物,即 Capnesterones A (1) 和 B (2),它们的 A 环具有交叉共轭的二烯酮结构单元。通过对红外光谱、ESIMS、1H NMR和13C NMR等光谱数据进行综合分析,并与以往文献进行对比,阐明了这两种化合物的结构。与 LPS 刺激的对照细胞相比,在 10 μM 的浓度下,类固醇 1 和 2 能降低 iNOS 水平(分别为 60% 和 82%),同时增加 COX-2 蛋白表达的释放量(分别为 134% 和 110%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
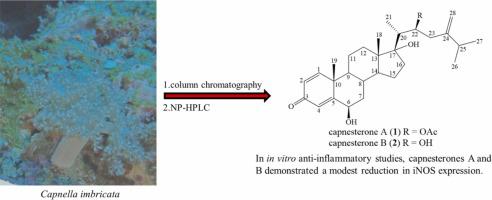
Capnesterones A and B, new steroids isolated from the soft coral Capnella imbricata
Two new non-withanolidal steroids with a cross-conjugated dienone structural unit in ring A, capnesterones A (1) and B (2), were isolated from the soft coral Capnella imbricata in the southeast waters of Taiwan. The compounds' structures were elucidated through a comprehensive analysis of spectroscopic data, including IR, ESIMS, 1H NMR, and 13C NMR, and through comparison with previous literature. At a concentration of 10 μM, steroids 1 and 2 exhibited reductions in iNOS levels (60 % and 82 %) compared to LPS-stimulated control cells, along with increased release (134 % and 110 %) against COX-2 protein expressions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry Letters
生物-生化与分子生物学
CiteScore
3.00
自引率
11.80%
发文量
190
审稿时长
34 days
期刊介绍:
Phytochemistry Letters invites rapid communications on all aspects of natural product research including:
• Structural elucidation of natural products
• Analytical evaluation of herbal medicines
• Clinical efficacy, safety and pharmacovigilance of herbal medicines
• Natural product biosynthesis
• Natural product synthesis and chemical modification
• Natural product metabolism
• Chemical ecology
• Biotechnology
• Bioassay-guided isolation
• Pharmacognosy
• Pharmacology of natural products
• Metabolomics
• Ethnobotany and traditional usage
• Genetics of natural products
Manuscripts that detail the isolation of just one new compound are not substantial enough to be sent out of review and are out of scope. Furthermore, where pharmacology has been performed on one new compound to increase the amount of novel data, the pharmacology must be substantial and/or related to the medicinal use of the producing organism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


