调节组织形态和功能的机械状态转换
IF 90.2
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
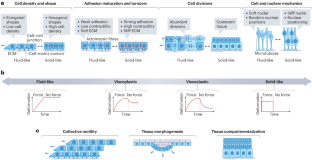
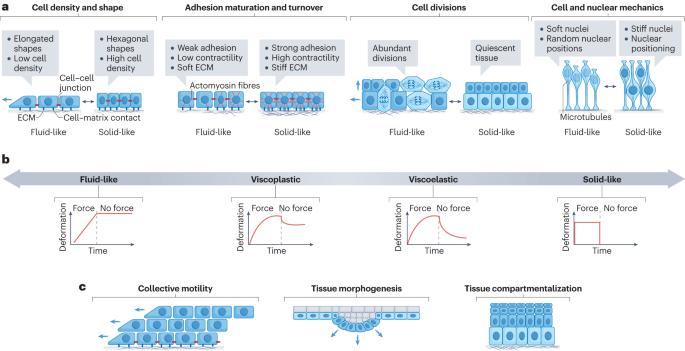
从胚胎发育、出生后生长、成人稳态到修复和疾病状态,细胞和组织在基因组活动、细胞命运、增殖、运动、新陈代谢和生长方面不断发生变化。重要的是,这些生物状态的转变与细胞和组织的机械和材料特性的变化相关联,称为机械状态转变。这些机械状态与物质、液体和固体的物理状态具有共同特征。组织可通过改变行为动力学或细胞间的连接性在不同机械状态之间切换。反过来,组织机械特性的这些变化已知可控制细胞和组织的功能,其中最重要的是细胞移动或组织变形的能力。因此,组织机械状态转换与跨生物长度和时间尺度的信息传递有关,尤其是在早期发育、伤口愈合和癌症等疾病的过程中。本综述将重点探讨组织尺度机械状态转换的生物学基础、它们如何从分子和细胞相互作用中产生,以及它们在生物体发育、平衡、再生和疾病中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanical state transitions in the regulation of tissue form and function
From embryonic development, postnatal growth and adult homeostasis to reparative and disease states, cells and tissues undergo constant changes in genome activity, cell fate, proliferation, movement, metabolism and growth. Importantly, these biological state transitions are coupled to changes in the mechanical and material properties of cells and tissues, termed mechanical state transitions. These mechanical states share features with physical states of matter, liquids and solids. Tissues can switch between mechanical states by changing behavioural dynamics or connectivity between cells. Conversely, these changes in tissue mechanical properties are known to control cell and tissue function, most importantly the ability of cells to move or tissues to deform. Thus, tissue mechanical state transitions are implicated in transmitting information across biological length and time scales, especially during processes of early development, wound healing and diseases such as cancer. This Review will focus on the biological basis of tissue-scale mechanical state transitions, how they emerge from molecular and cellular interactions, and their roles in organismal development, homeostasis, regeneration and disease. Tissues undergo changes in their mechanical and material properties through alterations in cytoskeleton organization, extracellular matrix adhesion and cell–cell connectivity. These mechanical state transitions orchestrate cell proliferation and movement and tissue growth during development, in adult tissue repair and in disease contexts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


