可视化伴侣介导的人类 20S 蛋白酶体多步骤组装
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
专用的组装因子协调着许多分子机器的逐步生产,包括介导蛋白质降解的 28 个亚基蛋白酶体核心颗粒(CP)。在这里,我们报告了七个重组人类亚复合物的冷冻电子显微镜重构,这些亚复合物在组装途径的广泛范围内可视化所有五个伴侣和三个活性位点肽。对这些与伴侣结合的中间产物和匹配的成熟蛋白酶体进行比较,揭示了决定亚基相继加入顺序的分子机制,以及蛋白酶体亚复合物和组装因子如何在亚基逐步加入后进行结构调整,以稳定中间产物,促进后续中间产物的形成,并最终重新排列以协调蛋白水解活化与活性位点的门控访问。这项研究为多蛋白复合物组装中间体的结构分析确立了方法学途径,阐明了组装因子的特定功能,揭示了人类蛋白酶体生物发生的基本概念原理,从而为之前的许多生物化学和遗传学观察提供了解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
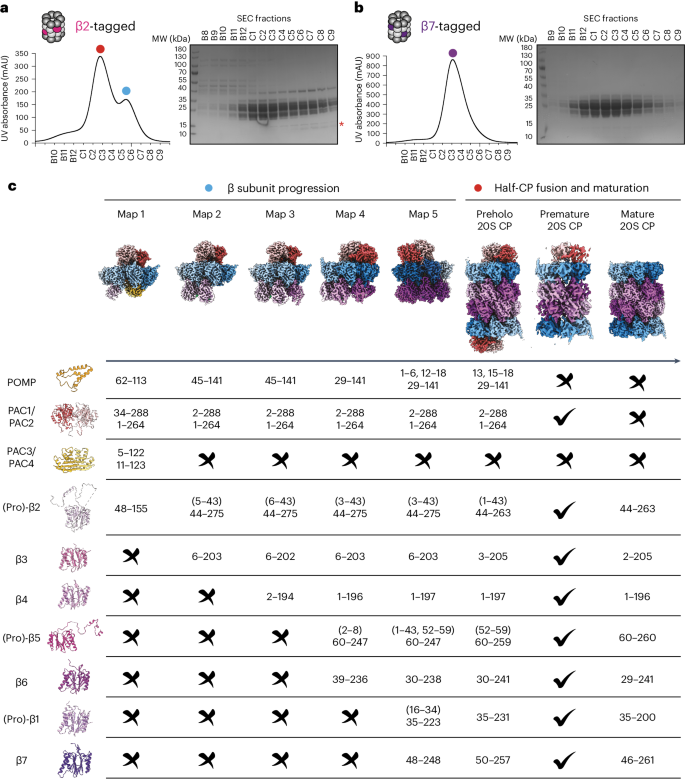
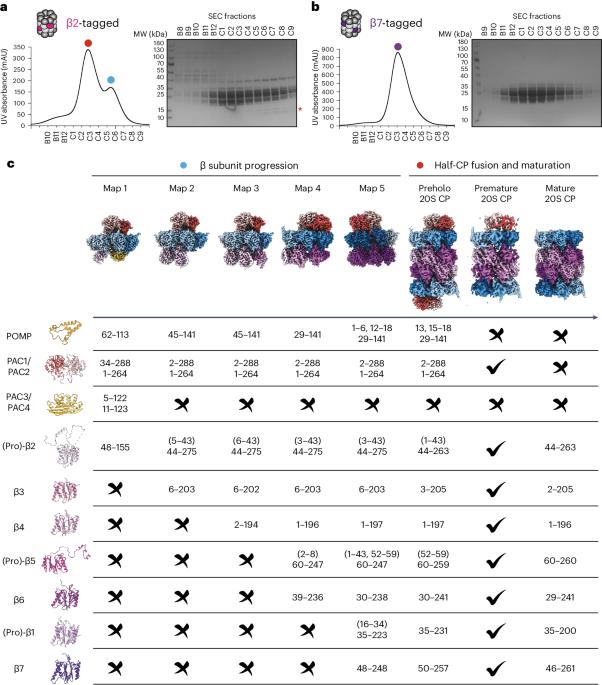
Visualizing chaperone-mediated multistep assembly of the human 20S proteasome
Dedicated assembly factors orchestrate the stepwise production of many molecular machines, including the 28-subunit proteasome core particle (CP) that mediates protein degradation. Here we report cryo-electron microscopy reconstructions of seven recombinant human subcomplexes that visualize all five chaperones and the three active site propeptides across a wide swath of the assembly pathway. Comparison of these chaperone-bound intermediates and a matching mature CP reveals molecular mechanisms determining the order of successive subunit additions, as well as how proteasome subcomplexes and assembly factors structurally adapt upon progressive subunit incorporation to stabilize intermediates, facilitate the formation of subsequent intermediates and ultimately rearrange to coordinate proteolytic activation with gated access to active sites. This work establishes a methodologic approach for structural analysis of multiprotein complex assembly intermediates, illuminates specific functions of assembly factors and reveals conceptual principles underlying human proteasome biogenesis, thus providing an explanation for many previous biochemical and genetic observations. Precise protease positioning and gating of the proteasome core require the ordered assembly of 28 subunits. Cryo-EM structures of seven intermediates visualize five dedicated chaperones and three propeptides mediating step-by-step assembly of the human 20S proteasome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


