碱基编辑人体器官组织中的膀胱生成遗传学揭示了多囊肾病的治疗策略
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
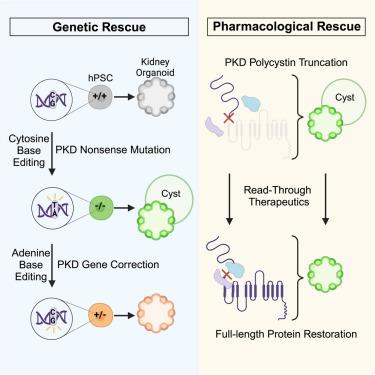
在多囊肾病(PKD)中,微小的肾小管会扩张成巨大的囊肿。作为世界上最常见的遗传疾病之一,PKD是通过杂合子功能缺失突变遗传的,但理论上需要额外的功能缺失。为了验证这一点,我们利用CRISPR碱基编辑技术建立了代表四种常见无义突变的等位基因系列人类多能干细胞。当分化成肾脏器官组织时,同源突变体会自发形成囊肿,而杂合突变体(原始突变体或碱基校正突变体)则不表达任何表型。利用这些突变体,我们发现真核核糖体选择性糖苷(ERSGs)可作为 PKD 治疗药物,使这些相同的无义突变获得核糖体读通。两种不同的ERSGs不仅能阻止囊肿的形成,还能通过部分恢复多囊卵巢素的表达来限制已形成囊肿的生长。此外,苷类还会在器官组织和小鼠的囊肿上皮中积累。我们的研究结果将人类多囊卵巢素阈值定义为一个可克服的药物靶点,可用于药物或基因治疗干预,对了解疾病机制和未来的临床试验具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Genetics of cystogenesis in base-edited human organoids reveal therapeutic strategies for polycystic kidney disease
In polycystic kidney disease (PKD), microscopic tubules expand into macroscopic cysts. Among the world’s most common genetic disorders, PKD is inherited via heterozygous loss-of-function mutations but is theorized to require additional loss of function. To test this, we establish human pluripotent stem cells in allelic series representing four common nonsense mutations, using CRISPR base editing. When differentiated into kidney organoids, homozygous mutants spontaneously form cysts, whereas heterozygous mutants (original or base corrected) express no phenotype. Using these, we identify eukaryotic ribosomal selective glycosides (ERSGs) as PKD therapeutics enabling ribosomal readthrough of these same nonsense mutations. Two different ERSGs not only prevent cyst initiation but also limit growth of pre-formed cysts by partially restoring polycystin expression. Furthermore, glycosides accumulate in cyst epithelia in organoids and mice. Our findings define the human polycystin threshold as a surmountable drug target for pharmacological or gene therapy interventions, with relevance for understanding disease mechanisms and future clinical trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


