预测纳米药物肿瘤蓄积的组织病理学生物标志物
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
癌症纳米药物的临床前景取决于有效的患者分层。在此,我们报告了纳米药物在肿瘤组织中积累的预测性生物标记物的鉴定结果。通过对纳米药物在小鼠肿瘤模型中的积累数据进行有监督的机器学习,我们发现血管密度和肿瘤相关巨噬细胞密度是关键的预测特征。在这两个特征的基础上,我们得出了与肿瘤中多柔比星脂质体浓度相关的生物标记物评分,并在免疫功能正常小鼠的三个合成肿瘤模型以及小鼠的四个细胞系衍生肿瘤和六个患者衍生肿瘤异种移植物中进行了验证。根据纳米药物的积累情况(高与低),该评分能有效区分肿瘤,接收器操作特征曲线下面积为 0.91。对 30 例患者肿瘤标本和 28 例相应的原发性肿瘤活检组织病理学评估证实,该评分能有效预测肿瘤对脂质体多柔比星的蓄积情况。纳米药物肿瘤蓄积的生物标志物可能有助于在癌症纳米药物临床试验中对患者进行分层。本文章由计算机程序翻译,如有差异,请以英文原文为准。
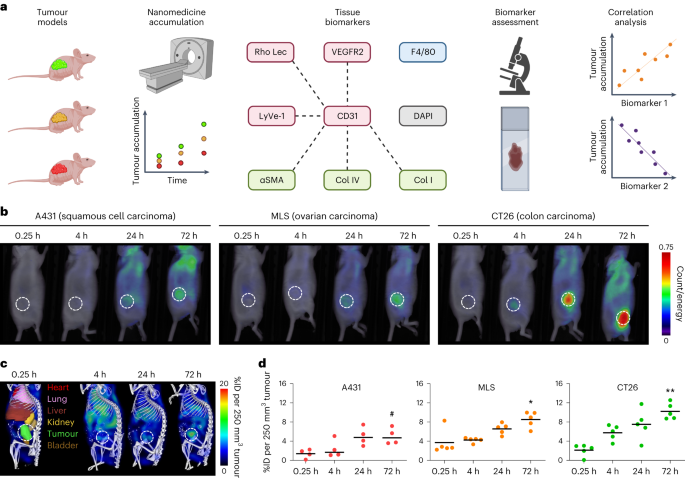
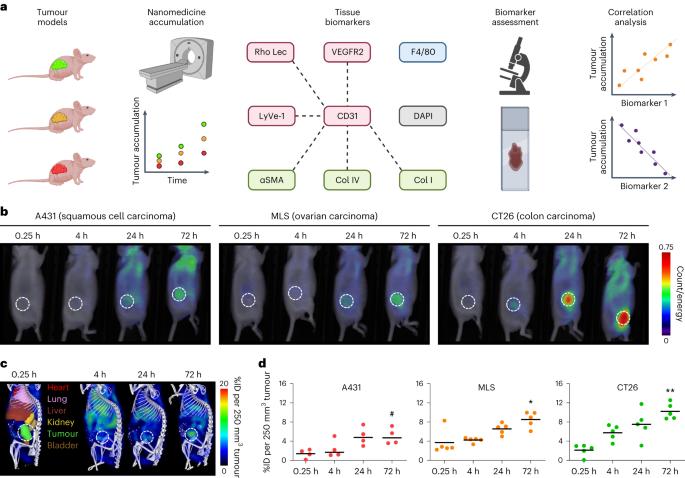
Histopathological biomarkers for predicting the tumour accumulation of nanomedicines
The clinical prospects of cancer nanomedicines depend on effective patient stratification. Here we report the identification of predictive biomarkers of the accumulation of nanomedicines in tumour tissue. By using supervised machine learning on data of the accumulation of nanomedicines in tumour models in mice, we identified the densities of blood vessels and of tumour-associated macrophages as key predictive features. On the basis of these two features, we derived a biomarker score correlating with the concentration of liposomal doxorubicin in tumours and validated it in three syngeneic tumour models in immunocompetent mice and in four cell-line-derived and six patient-derived tumour xenografts in mice. The score effectively discriminated tumours according to the accumulation of nanomedicines (high versus low), with an area under the receiver operating characteristic curve of 0.91. Histopathological assessment of 30 tumour specimens from patients and of 28 corresponding primary tumour biopsies confirmed the score’s effectiveness in predicting the tumour accumulation of liposomal doxorubicin. Biomarkers of the tumour accumulation of nanomedicines may aid the stratification of patients in clinical trials of cancer nanomedicines. The densities of blood vessels and of tumour-associated macrophages are key predictive features of the degree of accumulation of polymeric and liposomal nanomedicines, as shown for specimens of mouse and human tumours.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


