CHT1 对突触前高亲和性胆碱摄取的转运机制
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
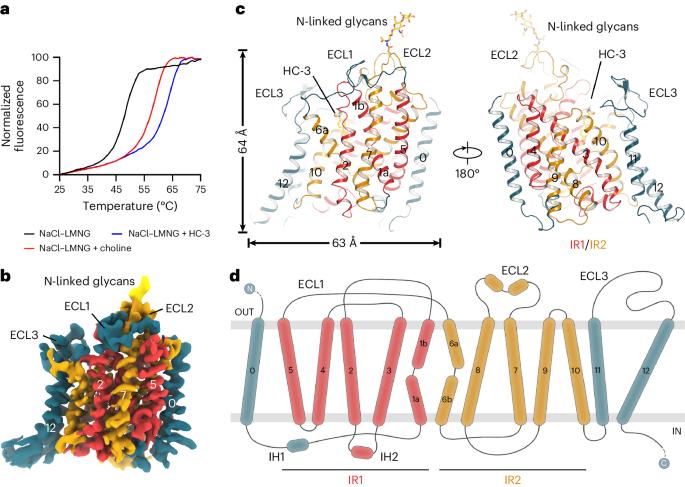
胆碱是一种重要的营养物质,也是乙酰胆碱(ACh)等重要代谢物生物合成的前体,在胎儿发育过程中,尤其是在大脑中发挥着核心作用。在胆碱能神经元中,高亲和性胆碱转运体(CHT1)提供了一种异常高效的再摄取机制,以重新利用突触内乙酰胆碱水解产生的胆碱,并维持突触前乙酰胆碱的合成。在这里,我们测定了人 CHT1 在三种不同状态下的结构:与竞争性抑制剂 hemicholinium-3 (HC-3) 结合的外向状态;与底物胆碱结合的内向闭锁状态;以及内向 apo 开放状态。我们的结构和功能特性阐明了抑制剂和底物是如何被识别的。此外,我们的发现还揭示了从外向状态过渡到内向状态时的构象变化,并建立了一个理解运输循环的框架,该循环依赖于细胞内短螺旋 IH1 对外向状态的稳定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transport mechanism of presynaptic high-affinity choline uptake by CHT1
Choline is a vital nutrient and a precursor for the biosynthesis of essential metabolites, including acetylcholine (ACh), that play a central role in fetal development, especially in the brain. In cholinergic neurons, the high-affinity choline transporter (CHT1) provides an extraordinarily efficient reuptake mechanism to reutilize choline derived from intrasynaptical ACh hydrolysis and maintain ACh synthesis in the presynapse. Here, we determined structures of human CHT1 in three discrete states: the outward-facing state bound with the competitive inhibitor hemicholinium-3 (HC-3); the inward-facing occluded state bound with the substrate choline; and the inward-facing apo open state. Our structures and functional characterizations elucidate how the inhibitor and substrate are recognized. Moreover, our findings shed light on conformational changes when transitioning from an outward-facing to an inward-facing state and establish a framework for understanding the transport cycle, which relies on the stabilization of the outward-facing state by a short intracellular helix, IH1. The authors report the structures of human CHT1 in the outward-open, inward-occluded and inward-open states, reveal the mechanism of HC-3 inhibition and choline recognition and elucidate the regulatory role of the intracellular helix IH1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


