工程 DNA 纳米材料显著降低阿尔茨海默氏症淀粉样蛋白-β的水平
IF 6.3
3区 综合性期刊
Q1 Multidisciplinary
引用次数: 0
摘要
虽然目前还没有有效的治疗方法来阻止或逆转阿尔茨海默病(AD)的进展,但一种有希望的治疗策略是降低淀粉样蛋白-β (a β)蛋白的水平,这种蛋白驱动淀粉样斑块的形成,淀粉样斑块是AD大脑的主要标志。在此,我们报告了两亲性脂质dna分子(LD)是通过将长烷基链纳入核苷酸碱基而设计的。在体内和体外显著下调阿尔茨海默病Aβ水平。与小分子化学药物和抗体疗法相比,组装的DNA纳米颗粒使它们能够有效地穿过血脑屏障(BBB)并在大脑中积累,从而增加了治疗效果。值得注意的是,脂质dna在体外显著下调了Aβ肽的水平。AD小鼠模型实验表明,LD治疗组表现出快速的认知行为改善,这与LD的大脑参与和a β水平降低有关。因此,分子工程DNA纳米材料可以有效地调节Aβ肽。这项工作可能为阿尔茨海默病的治疗提供一种有前途的DNA工程策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
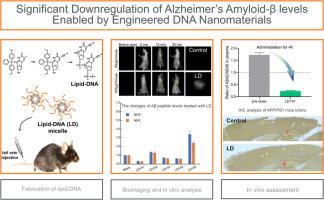
Significant downregulation of Alzheimer's amyloid-β levels enabled by engineered DNA nanomaterials
Although there are no effective therapies to block or reverse Alzheimer's disease (AD) progression at present, a promising therapeutic strategy is to reduce levels of amyloid-β (Aβ) proteins, which drive the formation of amyloid plaque, a primary hallmark in AD brains. Herein, we report that amphiphilic lipid-DNA molecules (LD) were designed by incorporating a long alkyl chain into the nucleotide base. It significantly down-regulated Alzheimer's Aβ levels in vivo and in vitro. In contrast to small-molecule chemical drugs and antibody therapies, the assembled DNA nanoparticles allowed them to effectively cross the blood-brain barrier (BBB) and accumulate in the brain, increasing the therapeutic effects. Notably, lipid-DNA downregulated the levels of Aβ peptides significantly in vitro. AD mice model experiments demonstrated that the LD-treated groups exhibited a rapid cognition behavioral improvement, which was associated with brain engagement of LD and reduced Aβ levels. Thus, the molecularly engineered DNA nanomaterials effectively regulated Aβ peptides. This work might provide a promising DNA engineering strategy for AD treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fundamental Research
Multidisciplinary-Multidisciplinary
CiteScore
4.00
自引率
1.60%
发文量
294
审稿时长
79 days
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


