重晶石-三水醋酸钠体系中的低温合成:AMPO4(A = Na、Li;M = Mn、Mn/Fe)中 M3+/M2+ 氧化还原偶的电化学活性
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过 NH4MPO4-H2O(M = Mn、Mn/Fe)与 AcONa-3H2O 或 AcOLi 的低温反应,分别分离出了相纯的 NaMPO4(M = Mn、Mn/Fe;与三苯基石同型)和 Li(Mn/Fe)PO4。在半电池中进行的电化学测试表明,与金属 Na 相比,Na 基化合物的电化学活性较差,而类似合成的 Li 对应物在 Na 电池中的循环性能良好。本文讨论了橄榄石家族相关成员的合成特征、晶体结构和性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
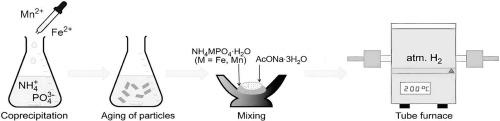
Low-temperature synthesis in the dittmarite–sodium acetate trihydrate system: electrochemical activity of M3+/M2+ redox couples in AMPO4 (A = Na, Li; M = Mn, Mn/Fe)
Phase-pure NaMPO4 (M = Mn, Mn/Fe; isotypic to triphylite) and Li(Mn/Fe)PO4 were isolated as a result of the low- temperature reaction between NH4MPO4·H2O (M = Mn, Mn/Fe) and AcONa·3H2O or AcOLi, respectively. Electrochemical tests in half-cells revealed that Na-based compounds exhibit poor electrochemical activity vs. metallic Na, while the similarly synthesized Li counterpart demonstrates decent cycling in Na cells. The synthetic features, crystal structures and properties of related members of the olivine family are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


