1-烷基-3-甲基咪唑卤化物水溶液渗透系数建模:反映离子和 ePC-SAFT 电结构的理论
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电解质溶液的热力学性质建模具有挑战性,但在各种应用中都需要。在这项工作中,我们探究了一种最新的电解质理论,该理论描述了离子内部电荷分布的细节。我们探索了这一理论的参数空间,以评估它是否有助于工程计算。为了检验这一理论,我们描述了 1-烷基-3-甲基咪唑卤化物水溶液中的渗透系数,这是一种具有不对称有机阳离子的离子液体。这些系统也借助两个版本的 ePC-SAFT EOS 进行了描述:"ePC-SAFT 修订版 "和 "ePC-SAFT 高级版"。这两个版本的参数都已估算出来。所研究离子液体水溶液的密度是在 298.15 K 和 101 kPa 条件下测量的。结果表明,"ePC-SAFT 高级版 "比 "ePC-SAFT 修订版 "运行得更好。新理论似乎相当灵活:对渗透系数的描述并不比 "ePC-SAFT 高级版 "差。我们还测试了电介质连续体中带电硬球系统的理论预测和计算机模拟。我们的研究结果推动了以本研究采用的理论方法为基础的模型的进一步发展和更广泛应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
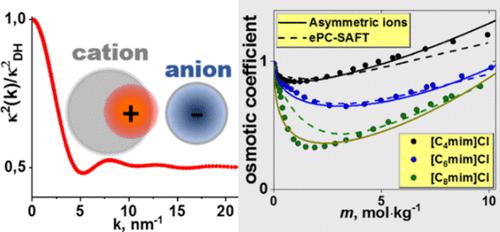
Modeling Osmotic Coefficients in Aqueous Solutions of 1-Alkyl-3-methylimidazolim Halides: A Theory That Reflects the Electrical Structure of Ions and ePC-SAFT
Modeling thermodynamic properties of electrolyte solutions is challenging but needed in various applications. In this work, we probe a recent electrolyte theory that describes details of the charge distribution inside ions. We explore the parameter space of this theory to assess whether it is helpful in engineering calculations. To test the theory, we describe the osmotic coefficients in aqueous solutions of 1-alkyl-3-methylimidazolium halides, ionic liquids that have asymmetric organic cations. These systems have also been described with the aid of two versions of the ePC-SAFT EOS: “ePC-SAFT Revised” and “ePC-SAFT Advanced”. Parameters for both versions have been estimated. The densities of aqueous solutions of the studied ionic liquids were measured at 298.15 K and 101 kPa. We show that the “ePC-SAFT Advanced” version works much better than the “ePC-SAFT Revised” version. The new theory appears to be quite flexible: the description of the osmotic coefficients is not inferior to that from “ePC-SAFT Advanced”. We also test predictions from theory versus computer simulation for a system of charged hard spheres in a dielectric continuum. Our results motivate further development and broader applications of the models based on the theoretical approach employed in this work.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


