开发通过烯胺环化制备 3-溴-N-烷基-1,6-萘啶酮的安全且可扩展的方法
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
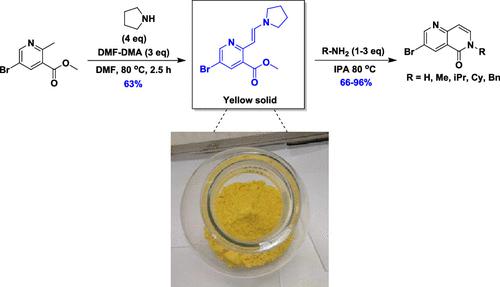
本文介绍了一种从伯胺制备 N-烷基-1,6-萘啶酮(N-alkyl-1,6-naphthyridones)的安全高效的方法。由于制备 N-烷基-1,6-萘啶酮的现有方法涉及强放热和苛刻试剂(三嗪/烷基卤化物),因此开发了一种避免使用这些有害试剂和条件的方法。所开发的路线包括制备一种稳定且可分离的固体关键烯胺中间体,该中间体可与伯胺发生环化反应,从而得到各种 N-烷基-1,6-萘啶酮,这些 N-烷基-1,6-萘啶酮在制备医药相关分子方面具有很高的价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development of a Safe and Scalable Method to Prepare 3-Bromo-N-alkyl-1,6-naphthyridones via Enamine Cyclization
A safe and efficient method to make N-alkyl-1,6-naphthyridones from primary amines is described. Due to issues with existing methodology involving strong exotherms and harsh reagents (triazine/alkyl halides), to prepare N-alkyl-1,6-naphthyridones, a method was developed that avoids these hazardous reagents and conditions. The developed route involved the preparation of a key enamine intermediate as a stable and isolatable solid, which could then undergo cyclization with primary amines to afford a variety of N-alkyl-1,6-naphthyridones that are of high interest in the preparation of pharmaceutically relevant molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


