以甲醇为可持续 C1 源,α-MoC 上的铂单原子和铂簇协同催化的无添加 N-甲基化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
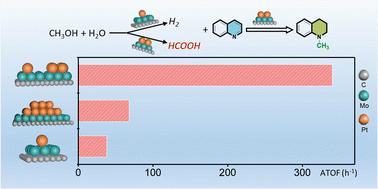
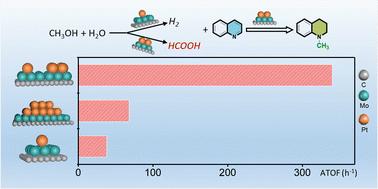
甲醇因其经济、丰富、无毒和可持续生产的特性,正被积极研究作为一种有前途的 C1 试剂,以取代有机合成中的传统 C1 试剂。然而,由于脱氢焓值较高,因此相关报道并不多见。在此,我们报告了铂单原子(Pt1)和铂团簇(Ptn)协同负载在 α-MoC 上,在甲醇水溶液中就能实现将各种不饱和含 N 化合物转化为高附加值的 N-甲基化化合物,成功避免了使用外部氢气或任何添加剂。在 Pt1+n/α-MoC 上共存的 Pt1 和 Ptn 之间的协同作用被认为是实现串联转化的必要条件,并具有优异的活性和选择性。Pt1/α-MoC 是水相甲醇重整原位制氢的活性位点,而 Ptn/α-MoC 则负责连续氢化和甲基化。由于含 N 化合物与铂颗粒(Ptn)的强配位会显著抑制催化活性,因此部分正 Ptnδ+ 物种对还原 N-甲基化反应至关重要。实验和 DFT 结果进一步表明,与甲醇通过中间体甲醛的传统借氢机制不同,甲酸是 N-甲基化反应的有效中间体和催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Additive-free N-methylation reaction synergistically catalyzed by Pt single atoms and clusters on α-MoC using methanol as a sustainable C1 source†
Methanol is being actively investigated as a promising C1 reagent to replace conventional C1 reagents in organic synthesis, due to its properties of being economical, abundant, nontoxic and sustainably producible. However, because of the high enthalpy of dehydrogenation, it has not been reported frequently. Herein, we report that Pt single atoms (Pt1) and Pt clusters (Ptn) are cooperatively loaded on α-MoC to achieve the conversion of various of unsaturated N-containing compounds into value-added N-methylation compounds simply in methanol aqueous solution, successfully avoiding the use of external hydrogen or any additives. The synergy between coexisting Pt1 and Ptn on Pt1+ n/α-MoC is identified as necessary to realize the tandem conversion with superior activity and selectivity. Pt1/α-MoC is the active site for in situ hydrogen production from aqueous phase methanol reforming, and Ptn/α-MoC is responsible for the successive hydrogenation and methylation. The partially positive Ptnδ + species are crucial for the reductive N-methylation reaction, as the strong coordination of N-containing compounds to Pt particles (Ptn) significantly inhibits the catalytic activity. Experimental and DFT results further show that, unlike the traditional hydrogen-borrowing mechanism of methanol via the intermediate formaldehyde, formic acid is found to be a potent intermediate and agent for N-methylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


