无水四氟化铈的合成
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
通过 CeF3 与 XeF2 反应,合成了无水 CeF4,并通过 XRD、SEM、FTIR、19F NMR 光谱法和热重分析法对其进行了研究。研究发现,CeF4 在空气中会发生水化反应,形成近似分子式为 [CeF4-0.2H2O]*0.7H2O 的结晶水合物。由于晶格中存在 OH...F 氢键,水分子既进入了铈的配位层,又与氟化铈(IV)形成了结晶水合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
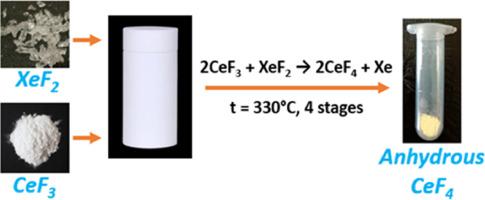
Synthesis of anhydrous cerium tetrafluoride
By reacting CeF3 with XeF2 the anhydrous CeF4 was synthesized and studied by XRD, SEM, FTIR, 19F NMR spectrometry and thermogravimetry. It was found that CeF4 undergoes hydration upon keeping in air forming the crystalline hydrate with the approximate formula [CeF4•0.2H2O]*0.7H2O. Water molecules both enter the coordination sphere of cerium and form crystalline hydrates with cerium(IV) fluoride due to OH…F hydrogen bonds in the crystal lattice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


