在化疗无效的转移性抗性前列腺癌患者中,醋酸阿比特龙与恩扎鲁胺的实际总生存率对比。
IF 5.1
2区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
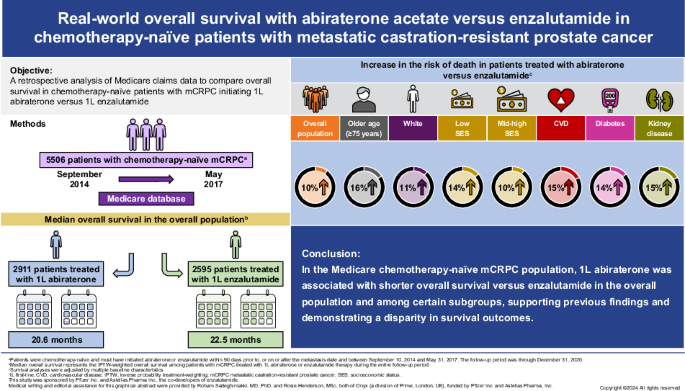
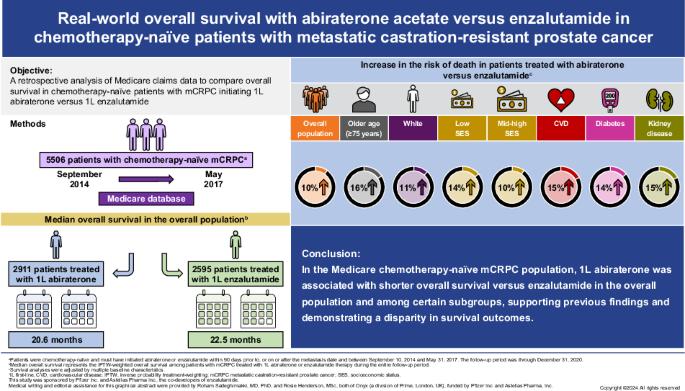
背景:目前还没有大型的头对头3期临床试验比较阿比特龙和恩扎鲁胺的总生存期(OS)。本研究利用医疗保险理赔数据,比较了化疗无效的转移性去势抵抗性前列腺癌(mCRPC)患者接受阿比特龙或恩杂鲁胺治疗后的OS:这项对医疗保险数据库(2009-2020年)的回顾性分析纳入了在指数期(2014年9月10日至2017年5月31日)开始一线(1L)阿比特龙或恩杂鲁胺治疗的前列腺癌索赔≥1次、转移性诊断、既往未接受过化疗或新型激素治疗的成年男性患者。采用逆概率治疗加权(IPTW)的Cox比例危险模型来比较阿比特龙和恩杂鲁胺治疗患者的OS,并对基线特征进行调整。此外,还根据基线特征进行了亚组分析:总共纳入了5506名接受1L阿比特龙(n = 2911)或恩扎鲁胺(n = 2595)治疗的患者。两组患者的中位随访时间相当(阿比特龙,19.1个月;恩扎鲁胺,20.3个月)。阿比特龙的IPTW调整后中位OS(95% CI)为20.6个月(19.7-21.4),恩杂鲁胺为22.5个月(21.2-23.8),IPTW调整后危险比(95% CI)为1.10(1.04-1.16)。在年龄≥75岁的患者、白人患者、基线糖尿病患者、心血管疾病患者、同时患有糖尿病和心血管疾病的患者、肾脏疾病患者以及所有社会经济阶层中,阿比特龙的中位OS明显短于恩杂鲁胺:结论:在医疗保险化疗无效的mCRPC人群中,1L阿比特龙与恩杂鲁胺相比,在总体人群中以及在年龄较大和合并症较多的亚群中,OS较差,这支持了之前真实世界研究的结果,并显示了结果上的差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Real-world overall survival with abiraterone acetate versus enzalutamide in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer
There are no large head-to-head phase 3 clinical trials comparing overall survival (OS) for abiraterone and enzalutamide. This study used Medicare claims data to compare OS in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) who initiated abiraterone or enzalutamide. This retrospective analysis of the Medicare database (2009–2020) included adult men with ≥1 claim for prostate cancer, metastatic diagnosis, and no prior chemotherapy or novel hormone therapy who initiated first-line (1L) abiraterone or enzalutamide in the index period (September 10, 2014 to May 31, 2017). Cox proportional-hazards models with inverse probability treatment-weighting (IPTW) were used to compare OS between abiraterone- and enzalutamide-treated patients, adjusting for baseline characteristics. Subgroup analyses by baseline characteristics were also conducted. Overall, 5506 patients who received 1L abiraterone (n = 2911) or enzalutamide (n = 2595) were included. Median follow-up was comparable in both cohorts (abiraterone, 19.1 months; enzalutamide, 20.3 months). IPTW-adjusted median OS (95% CI) was 20.6 months (19.7‒21.4) for abiraterone and 22.5 months (21.2‒23.8) for enzalutamide, with an IPTW-adjusted hazard ratio (95% CI) of 1.10 (1.04–1.16). Median OS was significantly shorter for abiraterone versus enzalutamide in patients ≥75 years old; White patients; patients with baseline diabetes, cardiovascular disease, both diabetes and cardiovascular disease, and renal disease; and across all socioeconomic strata. In the Medicare chemotherapy-naïve mCRPC population, 1L abiraterone was associated with worse OS versus enzalutamide in the overall population and among subgroups with older age and comorbidities, supporting findings from previous real-world studies and demonstrating a disparity in outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Prostate Cancer and Prostatic Diseases
医学-泌尿学与肾脏学
CiteScore
10.00
自引率
6.20%
发文量
142
审稿时长
6-12 weeks
期刊介绍:
Prostate Cancer and Prostatic Diseases covers all aspects of prostatic diseases, in particular prostate cancer, the subject of intensive basic and clinical research world-wide. The journal also reports on exciting new developments being made in diagnosis, surgery, radiotherapy, drug discovery and medical management.
Prostate Cancer and Prostatic Diseases is of interest to surgeons, oncologists and clinicians treating patients and to those involved in research into diseases of the prostate. The journal covers the three main areas - prostate cancer, male LUTS and prostatitis.
Prostate Cancer and Prostatic Diseases publishes original research articles, reviews, topical comment and critical appraisals of scientific meetings and the latest books. The journal also contains a calendar of forthcoming scientific meetings. The Editors and a distinguished Editorial Board ensure that submitted articles receive fast and efficient attention and are refereed to the highest possible scientific standard. A fast track system is available for topical articles of particular significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


