乳酸杆菌 Shirota 益生菌饮料可减少经常服用质子泵抑制剂的脊髓损伤患者的抗生素相关性腹泻:ECLISP 多中心 RCT 的亚组分析。
IF 2.1
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
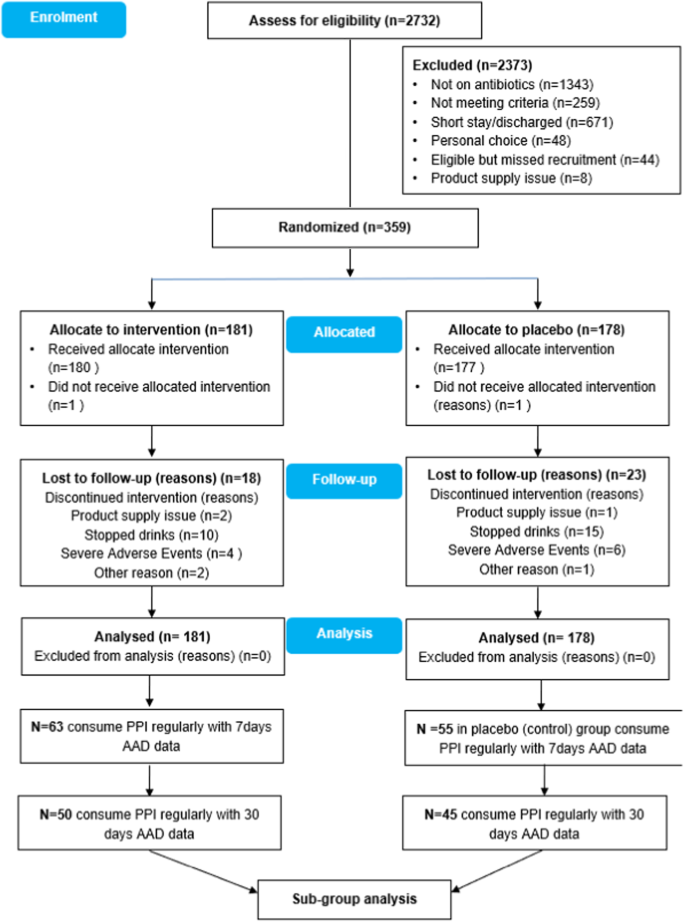
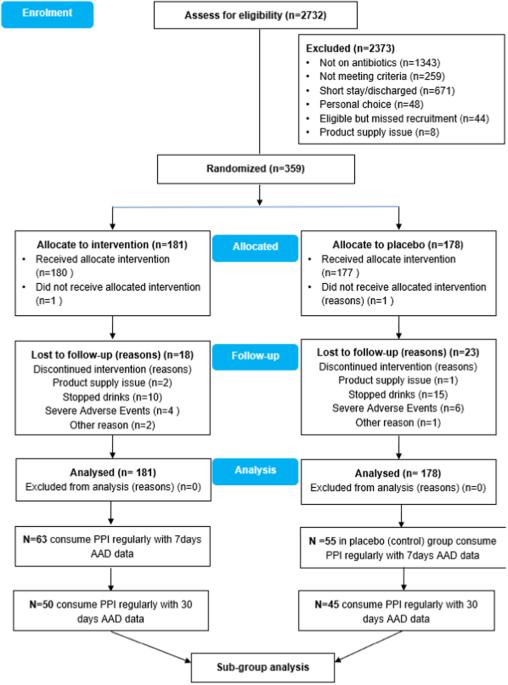
研究设计:这是一项多中心、随机、安慰剂对照、双盲试验(ECLISP 试验)的分组分析 目标: 评估至少含有 6.5 × 109 活白塔乳杆菌(LcS)的益生菌对预防抗生素相关性腹泻的疗效:评估含有至少 6.5 × 109 活的施罗塔乳杆菌(LcS)的益生菌在预防经常服用质子泵抑制剂(PPI)的脊髓损伤(SCI)患者出现抗生素相关性腹泻(AAD)方面的疗效。在抗生素疗程期间,每天服用一次 LcS 或安慰剂,之后继续服用 7 天。该试验的注册号为 ISRCTN:13119162:英国三家 SCI 中心(国家脊柱损伤中心、米德兰脊柱损伤中心和皇家公主脊髓损伤中心) 方法:2014 年 11 月至 2019 年 11 月期间,95 名符合条件且同意接受治疗的 SCI 患者(中位年龄:57 岁;智商范围:43-69)被随机分配接受 LcS(n = 50)或安慰剂(n = 45)治疗。主要结果是完成LcS/安慰剂治疗后30天内AAD的发生率:结果:抗生素疗程结束后 30 天内,LcS 组的 AAD 发生率明显较低(28.0 对 53.3%,RR:95% CI:0.53,0.31-0.89;z = 2.5,p = 0.01)。多变量逻辑回归分析表明,LcS 可降低 30 天后发生 AAD 的风险(OR:0.36,95% CI 0.13,0.99,p 结论:LcS 有可能预防 AAD 的发生:LcS有可能预防定期服用PPI的SCI患者中的弱势群体的AAD。需要进行确证性、随机、安慰剂对照研究,以确认这种明显的治疗效果,并将其转化为适当的临床结果:赞助商:养乐多本社株式会社本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lactobacillus casei Shirota probiotic drinks reduce antibiotic associated diarrhoea in patients with spinal cord injuries who regularly consume proton pump inhibitors: a subgroup analysis of the ECLISP multicentre RCT
This was a sub-group analysis of a multicentre, randomised, placebo-controlled, double-blind trial (ECLISP trial) To assess the efficacy of a probiotic containing at least 6.5 × 109 live Lactobacillus casei Shirota (LcS) in preventing antibiotic associated diarrhoea (AAD) in patients with spinal cord injury (SCI) who consumed proton pump inhibitor (PPI) regularly. LcS or placebo was given once daily for the duration of an antibiotic course and continued for 7 days thereafter. The trial was registered with ISRCTN:13119162. Three SCI centres (National Spinal Injuries Centre, Midland Centre for Spinal Injuries and Princess Royal Spinal Cord Injuries Centre) in the United Kingdom Between November 2014, and November 2019, 95 eligible consenting SCI patients (median age: 57; IQ range: 43-69) were randomly allocated to receive LcS (n = 50) or placebo (n = 45). The primary outcome is the occurrence of AAD up to 30 days after finishing LcS/placebo. The LcS group had a significantly lower incidence of AAD at 30 days after finishing the antibiotic course (28.0 v 53.3%, RR: 95% CI: 0.53, 0.31–0.89; z = 2.5, p = 0.01). Multivariate logistic regression analysis identified that LcS can reduce the risk of AAD at 30 days (OR: 0.36, 95% CI 0.13, 0.99, p < 0.05). No intervention-related adverse events were reported during the study. LcS has the potential to prevent AAD in what could be considered a defined vulnerable group of SCI patients on regular PPI. A confirmatory, randomised, placebo-controlled study is needed to confirm this apparent therapeutic success to translate it into appropriate clinical outcomes. Yakult Honsha Co., Ltd.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


