TBAB 催化对位醌甲化物的 1,6-共轭重氮化反应:获得多取代α-重氮羰基化合物的有效途径
IF 5.8
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过溴化四丁基铵(TBAB)催化的 1,6 共轭加成途径,建立了重氮乙酸酯与对醌甲酯的高效重氮化反应。该方法提供了一种方便、安全和快速的途径来生成多种多取代的 α-重氮羰基化合物,具有良好的官能团耐受性、高原子经济性和易得性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
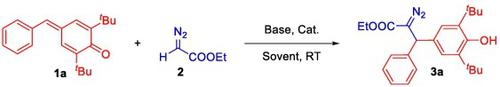
TBAB-catalyzed 1,6-conjugate diazotization of para-quinone methides: A very effective access to polysubstituted α-diazocarbonyl compounds
A highly efficient diazotization of diazoacetates with para-quinone methides has been established via a tetrabutyl ammonium bromide (TBAB)-catalyzed 1,6-conjugated addition pathway. This methodology affords a convenient, safe, and rapid way to generating diverse polysubstituted α-diazocarbonyl compounds, displaying good functional group tolerance, high atom economy, and easy accessibility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Saudi Chemical Society
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
8.90
自引率
1.80%
发文量
120
审稿时长
38 days
期刊介绍:
Journal of Saudi Chemical Society is an English language, peer-reviewed scholarly publication in the area of chemistry. Journal of Saudi Chemical Society publishes original papers, reviews and short reports on, but not limited to:
•Inorganic chemistry
•Physical chemistry
•Organic chemistry
•Analytical chemistry
Journal of Saudi Chemical Society is the official publication of the Saudi Chemical Society and is published by King Saud University in collaboration with Elsevier and is edited by an international group of eminent researchers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


