二氧化碳在 H2O 中的扩散性:块状和封闭状态下的实验研究和分子模拟综述
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二氧化碳在 H2O 中的扩散是碳捕获和储存、提高石油采收率以及食品工业等重要工业和环境过程中的一个核心特性,本文对二氧化碳在 H2O 中扩散的现有实验和分子模拟研究进行了深入综述。研究涵盖了散装和封闭系统的情况。对收集到的实验和分子模拟数据进行了分析,并设计了简单且计算效率高的相关关系。这些相关性适用于从 273 K 和 0.1 MPa 到 473 K 和 45 MPa 的条件。此外,还收集了二氧化碳在盐水中扩散系数的现有实验数据,并详细研究了它们与温度、压力和盐度的关系。此外,还介绍了文献中报道的其他工程模型和相关性。模拟研究回顾的重点是力场组合、低压和高压下的扩散系数数据、有限尺寸效应以及基于分子动力学数据开发的相关性。关于密闭系统,我们回顾了测量和计算密闭二氧化碳扩散率的主要方法,并讨论了主要的天然和人工密闭介质(即霞石、方解石、硅石、MOFs 和碳材料)。详细讨论了封闭条件下 CO2 和 H2O 扩散的驱动力,以及 H2O 吸附在亲水封闭介质上等效应对 CO2 扩散性的作用。最后,还展望了未来的研究方向,以推动在体相和约束条件下二氧化碳在 H2O 中的扩散。本文章由计算机程序翻译,如有差异,请以英文原文为准。
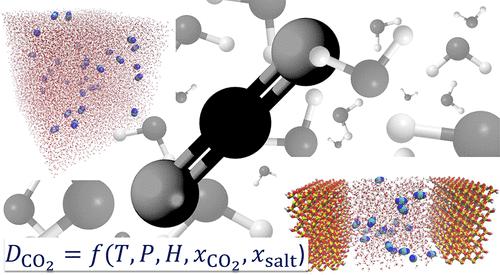
Diffusivity of CO2 in H2O: A Review of Experimental Studies and Molecular Simulations in the Bulk and in Confinement
An in-depth review of the available experimental and molecular simulation studies of CO2 diffusion in H2O, which is a central property in important industrial and environmental processes, such as carbon capture and storage, enhanced oil recovery, and in the food industry is presented. The cases of both bulk and confined systems are covered. The experimental and molecular simulation data gathered are analyzed, and simple and computationally efficient correlations are devised. These correlations are applicable to conditions from 273 K and 0.1 MPa up to 473 K and 45 MPa. The available experimental data for diffusion coefficients of CO2 in brines are also collected, and their dependency on temperature, pressure, and salinity is examined in detail. Other engineering models and correlations reported in literature are also presented. The review of the simulation studies focuses on the force field combinations, the data for diffusivities at low and high pressures, finite-size effects, and the correlations developed based on the Molecular Dynamics data. Regarding the confined systems, we review the main methods to measure and compute the diffusivity of confined CO2 and discuss the main natural and artificial confining media (i.e., smectites, calcites, silica, MOFs, and carbon materials). Detailed discussion is provided regarding the driving force for diffusion of CO2 and H2O under confinement, and on the role of effects such as H2O adsorption on hydrophilic confining media on the diffusivity of CO2. Finally, an outlook of future research paths for advancing the field of CO2 diffusivity in H2O at the bulk phase and in confinement is laid out.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


