生物乙醇转化制氢和丙烯催化过程的热力学和动力学分析
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究表明,乙醇蒸汽转化(SRE)或乙醇转化为丙烯(ETP)过程的初始反应途径可能相同,包括脱氢和缩合反应,并形成乙醛和丙酮。与 SRE 不同,ETP 工艺中丙烯和氢气的平衡产率与温度和乙醇浓度的关系不大。根据含碳化合物的选择性,提出了一个确定氢产率的方程式,根据该方程式可以计算出 SRE 和 ETP 工艺产物中的氢浓度。本文章由计算机程序翻译,如有差异,请以英文原文为准。

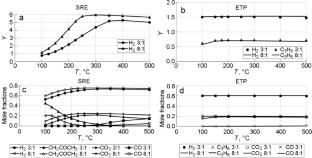
Thermodynamic and Kinetic Analysis of Catalytic Processes of Bioethanol Conversion for Hydrogen and Propylene Obtaining
It is shown that the initial reaction pathways of the processes of steam reforming of ethanol (SRE) or ethanol to propylene (ETP) conversion can be the same and include dehydrogenation and condensation reactions with the formation of acetaldehyde and acetone. The equilibrium yields of propylene and hydrogen in the ETP process, in contrast to SRE, depend little on both temperature and ethanol concentration. An equation for the determination of hydrogen yield as the function of selectivity of carbon-containing compounds has been proposed, based on which the hydrogen concentration in products of the SRE and ETP processes can be calculated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


