超基性岩中铁青金石氧化作用低温产生 H2 的动力学过程
IF 3.7
1区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
(Mg,Fe)(OH)2铁青石是超基性岩中低温(423 K)非生物产生H2的潜在候选矿物之一。为了验证这一假设,在 348 至 573 K 的温度范围内,将粒度与天然样品(40-100 nm)相似的合成铁青质青金石与纯水反应。该反应在 378 K 下 ∼ 2 个月内完成,属于热活化反应,活化能为 145 ± 1 kJ/mol。根据实验数据集完善了铁石棉(Fe(OH)2)的标准状态形成焓和第三定律熵。新的热力学参数表明,在氢活度比以前计算的低得多的情况下,铁青石是稳定的。铁青云母的蚀变产生 H2 的速率与目前在自然环境(蛇绿混杂岩和大洋中脊)中观测到的 H2 释放速率相一致。然而,这需要高效的流体更新,而不是通过橄榄石蛇绿岩化产生 H2,后者可以在静态水力条件下进行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
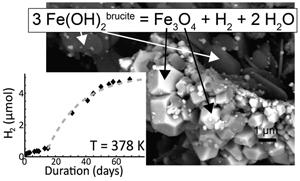
Kinetics of low-temperature H2 production in ultramafic rocks by ferroan brucite oxidation
Ferroan brucite, (Mg,Fe)(OH)2, is among the potential mineral candidates for low temperature (<423 K) abiotic H2 production in ultramafic rocks. To verify this assumption, synthetic ferroan brucite with grain size similar to that observed in natural samples (40–100 nm) was reacted with pure water at temperatures ranging from 348 to 573 K. Experimental products are consistent with the reaction 3 Fe(OH)2brucite = Fe3O4 + H2 + 2 H2O. This reaction reached completion in ∼2 months at 378 K and is thermally activated with an activation energy of 145 ± 1 kJ/mol. The standard state formation enthalpy and the third law entropy of amakinite, Fe(OH)2, were refined from the experimental dataset. The new thermodynamic parameters imply that ferroan brucite is stable at significantly lower hydrogen activity than previously calculated. The alteration of Fe-brucite produces H2 at rates compatible with present day observations of H2 emissions in natural settings (ophiolite and mid-oceanic ridges). However, efficient fluid renewal is required, as opposed to H2 production through olivine serpentinisation, which can proceed in static hydraulic conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Geochemical Perspectives Letters
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
7.00
自引率
2.00%
发文量
42
审稿时长
15 weeks
期刊介绍:
Geochemical Perspectives Letters is an open access, internationally peer-reviewed journal of the European Association of Geochemistry (EAG) that publishes short, highest-quality articles spanning geochemical sciences. The journal aims at rapid publication of the most novel research in geochemistry with a focus on outstanding quality, international importance, originality, and stimulating new developments across the vast array of geochemical disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


