聚(3-己基噻吩)和聚(乙烯基邻苯二酚)段的粘合嵌段共聚物的单击合成
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
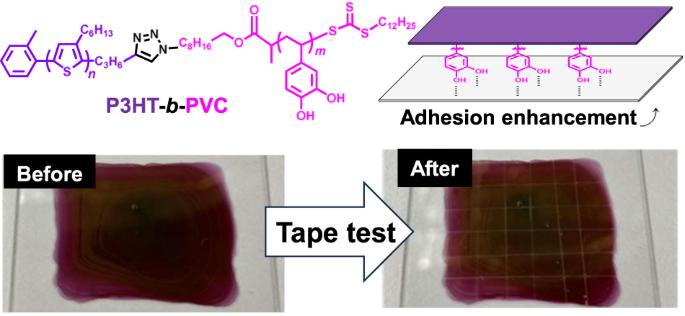
半导体聚合物引起了研究人员的极大关注,因为这些聚合物可能适用于柔性/可拉伸有机电子产品。然而,尽管这些材料在基底上的粘附性是一个重要特性,但却很少有人对此进行研究。在此,我们合成了一种新型嵌段共聚物--聚(3-己基噻吩)-b-聚(乙烯基邻苯二酚)(P3HT-b-PVC),并对其进行了表征,该聚合物具有更好的粘附性能。P3HT-b-PVC 是通过铜催化叠氮-炔烃环加成反应成功合成的,该反应是在链端官能化的带有炔烃基(P3HT-Alkyne)的 P3HT 和带有叠氮基(PSVC-Azide)的链端官能化的聚(3,4-二叔丁基二甲基硅氧基苯乙烯)之间进行的,然后对 PSVC-Azide 部分的叔丁基二甲基硅氧基基团进行脱保护。胶带测试结果表明,P3HT-b-PVC 薄膜的粘附性能大大优于相应的 P3HT 薄膜。此外,尽管在 P3HT-b-PVC 中存在绝缘 PVC 块,P3HT-b-PVC 薄膜仍表现出 1.1 × 10-5 cm2V-1s-1 的孔迁移率,与相应的 P3HT 薄膜(1.8 × 10-5 cm2V-1s-1)相当。据我们所知,这是首次阐明基于 P3HT 的嵌段共聚物的原始粘附特性和电荷迁移率的研究。所提出的合成方法可扩展用于开发具有其他 π 共轭聚合物段的各种嵌段共聚物,为需要高粘合性材料的各种应用提供新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
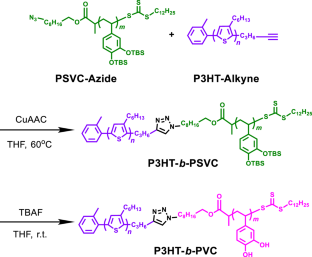
Click synthesis of an adhesive block copolymer with poly(3-hexylthiophene) and poly(vinyl catechol) segments
Semiconducting polymers have garnered considerable attention from researchers, as these polymers are potentially applicable to flexible/stretchable organic electronics. However, although the adhesiveness of these materials on substrates is an important characteristic, it has rarely been investigated. Herein, we synthesized and characterized a novel block copolymer, poly(3-hexylthiophene)-b-poly(vinyl catechol) (P3HT-b-PVC), with improved adhesion properties. P3HT-b-PVC was successfully synthesized via a copper-catalyzed azide–alkyne cycloaddition reaction between chain-end-functionalized P3HT with an alkyne group (P3HT-Alkyne) and chain-end-functionalized poly(3,4-di-tert-butyldimethylsilyloxystyrene) with an azide group (PSVC-Azide), followed by deprotection of tert-butyldimethylsilyloxy groups from the PSVC-Azide segment. Tape test results showed that the adhesion property of the P3HT-b-PVC film was considerably better than that of the corresponding P3HT film. Furthermore, despite the presence of an insulating PVC block in P3HT-b-PVC, the P3HT-b-PVC thin film exhibited a hole mobility of 1.1 × 10−5 cm2V−1s−1, which was comparable to that of the corresponding P3HT thin film (1.8 × 10−5 cm2V−1s−1). To the best of our knowledge, this is the first study to elucidate the primitive adhesion properties and charge mobility of P3HT-based block copolymers. The proposed synthetic approach may be extended to develop various block copolymers with other π-conjugated polymer segments, providing new avenues for various applications that require highly-adhesive materials. A novel block copolymer, poly(3-hexylthiophene)-b-poly(vinyl catechol) (P3HT-b-PVC) was successfully synthesized via a Click reaction between chain-endfunctionalized P3HT with an alkyne group (P3HT-Alkyne) and chain-end-functionalized poly(3,4-di-tert-butyldimethylsilyloxystyrene) with an azide group (PSVC-Azide), followed by deprotection of tert-butyldimethylsilyloxy groups from the PSVC-Azide segment. Tape test results showed that the adhesion property of the P3HT-b-PVC film was considerably better than that of the corresponding P3HT film. Furthermore, despite the presence of an insulating PVC block in P3HT-b-PVC, the P3HT-b-PVC thin film exhibited a hole mobility comparable to that of the corresponding P3HT thin film.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


