通过基本亮氨酸拉链结构域构建用于植物基因转染的肽/质粒 DNA 复合物
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
植物基因改造的一个重要方法是利用肽/pDNA 复合物将基因转移到植物细胞中。使用传统的载体肽时,肽序列必须含有大量阳离子氨基酸才能凝结并导入 pDNA。因此,pDNA 从复合物中解离的效率很低,经常会造成问题。在这里,我们设计了一种新的肽载体,它模仿了 DNA 结合蛋白的碱性亮氨酸拉链(bZIP)结构域,其中 (LU)4 是亮氨酸拉链结构域,(KUA)3 是碱性 DNA 结合和细胞穿透结构域(U = α-氨基异丁酸)。(KUA)3-(LU)4肽与pDNA混合后,DNA分子凝结成约130纳米的纳米颗粒。此外,将(KUA)3-(LU)4 多肽和 pDNA 复合物导入拟南芥(A. thaliana)叶片后,可在植物细胞中检测到报告蛋白的表达。因此,模仿 bZIP 结构域的 (KUA)3-(LU)4 多肽是一种新型高效的 pDNA 载体,具有很高的解离效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
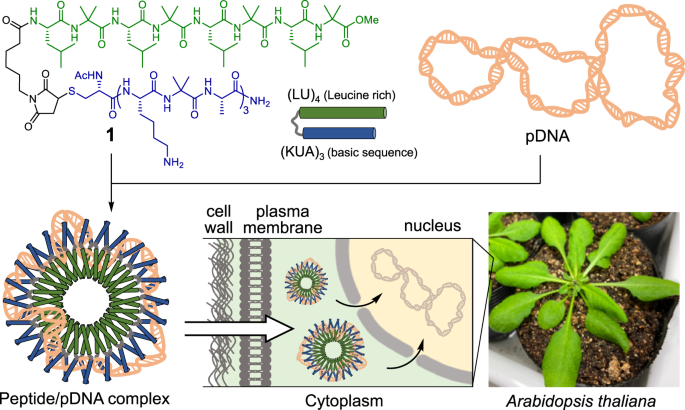
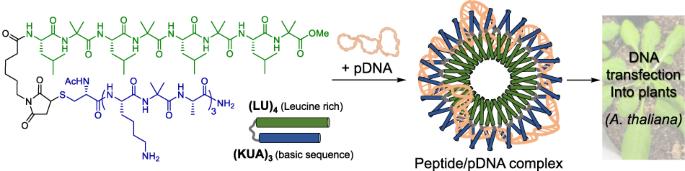
Construction of peptide/plasmid DNA complexes for plant gene transfection via the basic leucine zipper domain
An important method for plant genetic modification is using peptide/pDNA complexes to transfer genes into plant cells. With conventional carrier peptides, the peptide sequence must contain a high amount of cationic amino acids to condense and introduce pDNA. As a result, the dissociation of pDNA from the complex is inefficient, often causing problems. Herein, we designed a new peptide carrier that mimics the basic leucine zipper (bZIP) domain of DNA-binding proteins, in which (LU)4 is the leucine zipper motif and (KUA)3 is the basic DNA-binding and cell-penetrating motif (U = α-aminoisobutyric acid). After (KUA)3-(LU)4 peptide was mixed with pDNA, DNA molecules were condensed to form nanoparticles of approximately 130 nm. Furthermore, when complexes of (KUA)3-(LU)4 peptide and pDNA were introduced into the leaves of Arabidopsis thaliana (A. thaliana), expression of the reporter protein was detected in the plant cells. Thus, (KUA)3-(LU)4 peptide that mimics the bZIP domain is a novel and efficient carrier for pDNA with high dissociation efficiency. A new peptide carrier that mimics the basic leucine zipper domain (bZIP) of DNA-binding proteins was designed, in which (LU)4 is the leucine zipper motif and (KUA)3 is the basic DNA-binding motif (U = α-aminoisobutyric acid). When mixed with pDNA, (KUA)3-(LU)4 peptide condensed DNA molecules to form nanoparticles. Furthermore, when complexes of the (KUA)3-(LU)4 peptide and pDNA were introduced into the leaves of Arabidopsis thaliana (A. thaliana), the reporter protein was expressed in plant cells. Thus, (KUA)3-(LU)4 is an efficient carrier of pDNA with high dissociation efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


