基于生物标志物的阿尔茨海默病分期:原理与临床应用。
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
疾病分期,即通过大脑病理的空间范围和负荷来估计阿尔茨海默病(AD)的严重程度,是阿尔茨海默病金标准神经病理学诊断的关键。目前的AD体内诊断框架是基于脑脊液中淀粉样蛋白-β和tau的异常浓度或正电子发射计算机断层扫描,而分子成像技术的突破为AD的体内分期提供了可能。本综述侧重于多个医学领域共有的疾病分期关键原则,强调了AD体内分期的潜力,以改变我们对临床前AD的认识,完善疾病改变疗法试验的入选标准,并在抗淀粉样蛋白疗法时代帮助临床决策。我们对最近基于生物标志物的AD分期系统进行了最前沿的回顾,并强调了它们对理解AD自然史的贡献。此外,我们还概述了使用更易获得的体液生物标志物来分期 AD 严重程度的假设框架。此外,通过将基于淀粉样蛋白 PET 的分期应用于最近发表的抗淀粉样蛋白治疗试验,我们强调了基于生物标志物的疾病分期框架如何能够说明轻度症状 AD 患者身上已经发生的众多病理变化。最后,我们讨论了与疾病分期的验证和标准化相关的挑战,并对潜在的临床应用提供了前瞻性的观点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
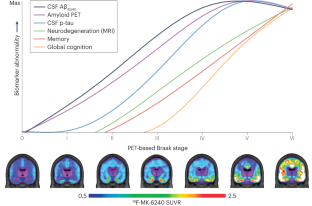
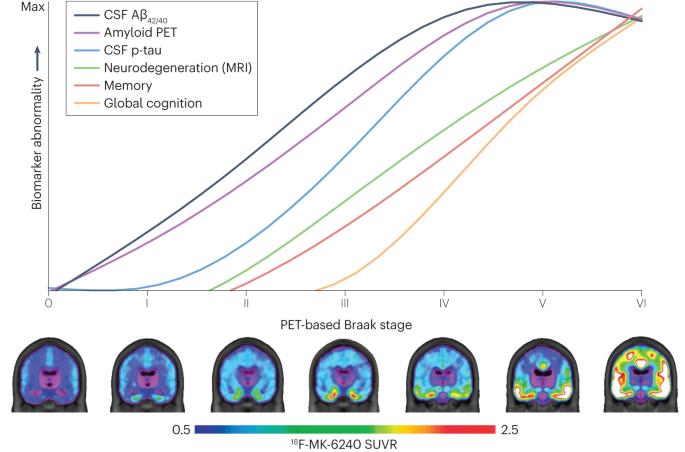
Biomarker-based staging of Alzheimer disease: rationale and clinical applications
Disease staging, whereby the spatial extent and load of brain pathology are used to estimate the severity of Alzheimer disease (AD), is pivotal to the gold-standard neuropathological diagnosis of AD. Current in vivo diagnostic frameworks for AD are based on abnormal concentrations of amyloid-β and tau in the cerebrospinal fluid or on PET scans, and breakthroughs in molecular imaging have opened up the possibility of in vivo staging of AD. Focusing on the key principles of disease staging shared across several areas of medicine, this Review highlights the potential for in vivo staging of AD to transform our understanding of preclinical AD, refine enrolment criteria for trials of disease-modifying therapies and aid clinical decision-making in the era of anti-amyloid therapeutics. We provide a state-of-the-art review of recent biomarker-based AD staging systems and highlight their contributions to the understanding of the natural history of AD. Furthermore, we outline hypothetical frameworks to stage AD severity using more accessible fluid biomarkers. In addition, by applying amyloid PET-based staging to recently published anti-amyloid therapeutic trials, we highlight how biomarker-based disease staging frameworks could illustrate the numerous pathological changes that have already taken place in individuals with mildly symptomatic AD. Finally, we discuss challenges related to the validation and standardization of disease staging and provide a forward-looking perspective on potential clinical applications. Recent clinical trials have highlighted the need for Alzheimer disease (AD) staging rather than simply noting the presence or absence of AD pathology. This article reviews current biomarker-based AD staging systems and outlines hypothetical frameworks to stage AD severity using fluid biomarkers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


