肝细胞癌的辅助和新辅助免疫疗法。
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
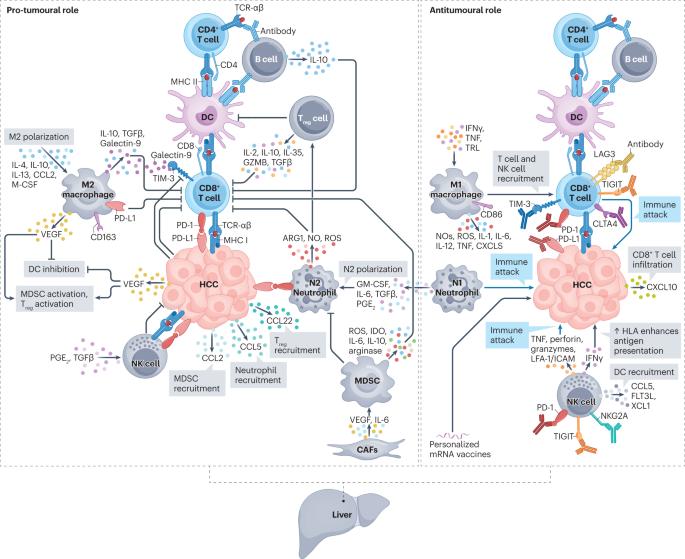
肝癌,特别是肝细胞癌(HCC),是全球第六大常见癌症和第三大癌症死因。有效的系统性疗法,尤其是涉及免疫检查点抑制剂(ICIs)的疗法的开发,大大改善了晚期 HCC 患者的预后。约有 30% 的患者被诊断为早期疾病,目前正在接受可能治愈的疗法,如切除术、肝移植或局部消融术,这些疗法可使患者的中位总生存期超过 60 个月。尽管如此,这些患者中仍有高达 70% 的患者会在切除术或局部消融术后 5 年内复发。迄今为止,对 HCC 患者进行辅助治疗的随机临床试验结果均为阴性。IMbrave 050 试验满足了这一主要的未满足需求,该试验显示,切除术或局部消融术后复发风险较高的患者接受阿特珠单抗加贝伐单抗的辅助治疗后,可获得无复发生存期。与此同时,对早期患者单独或联合使用新辅助 ICIs 的研究也显示了疗效。在本综述中,我们全面概述了目前治疗早期 HCC 患者的方法。我们还描述了肿瘤免疫微环境以及 ICIs 和癌症疫苗在这种情况下的作用机制。最后,我们总结了新辅助疗法和辅助疗法 II/III 期试验的现有证据,并讨论了新出现的临床试验、生物标志物的确定以及未来研究的临床试验设计注意事项。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
Liver cancer, specifically hepatocellular carcinoma (HCC), is the sixth most common cancer and the third leading cause of cancer mortality worldwide. The development of effective systemic therapies, particularly those involving immune-checkpoint inhibitors (ICIs), has substantially improved the outcomes of patients with advanced-stage HCC. Approximately 30% of patients are diagnosed with early stage disease and currently receive potentially curative therapies, such as resection, liver transplantation or local ablation, which result in median overall survival durations beyond 60 months. Nonetheless, up to 70% of these patients will have disease recurrence within 5 years of resection or local ablation. To date, the results of randomized clinical trials testing adjuvant therapy in patients with HCC have been negative. This major unmet need has been addressed with the IMbrave 050 trial, demonstrating a recurrence-free survival benefit in patients with a high risk of relapse after resection or local ablation who received adjuvant atezolizumab plus bevacizumab. In parallel, studies testing neoadjuvant ICIs alone or in combination in patients with early stage disease have also reported efficacy. In this Review, we provide a comprehensive overview of the current approaches to manage patients with early stage HCC. We also describe the tumour immune microenvironment and the mechanisms of action of ICIs and cancer vaccines in this setting. Finally, we summarize the available evidence from phase II/III trials of neoadjuvant and adjuvant approaches and discuss emerging clinical trials, identification of biomarkers and clinical trial design considerations for future studies. Patients with early stage hepatocellular carcinoma typically undergo resection, liver transplantation or local ablation; however, 30–50% will have disease recurrence at 3 years. The authors of this Review describe the tumour immune microenvironment and mechanism of action of immunotherapies, and discuss the available evidence from phase II/III trials of neoadjuvant and adjuvant treatment approaches in this setting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


