结构对 Pt-Sn-DeAlBEA 用于丙烷脱氢的活性、选择性和稳定性的影响
摘要
最近的研究发现,脱铝沸石 BEA(DeAlBEA)是一种极具吸引力的载体,可用于分散铂和铂硒,作为丙烷脱氢(PDH)的催化剂。在本研究中,我们报告了 Pt-Sn-DeAlBEA 催化剂的制备、结构表征和 PDH 活性与 Pt/Al 比(此处 Al 代表母体沸石 H-BEA 中的 Al 含量)的函数关系。支持物 Sn-DeAlBEA 是通过在 DeAlBEA 中加入 Sn 制备的。通过 X 射线吸收光谱 (XAS) 和紫外-可见光谱对这种材料进行表征,发现 Sn 以 Sn(IV) 阳离子的形式结合到 BEA 框架中。以 Pt/Al 比率(0.001-0.026)制备了 Pt-Sn-DeAlBEA 催化剂,并通过吸附探针分子的红外(IR)光谱和 XAS 对其进行了表征,以了解 Pt-Sn-DeAlBEA 中铂负载量变化对铂结构的影响。在 DeAlBEA(即 Pt-DeAlBEA)上的铂分散产生了铂-铂平均配位数为 9(25 ∼ Å)的铂纳米粒子,铂/铝比率为 0.001 及以上。相反,在 Sn-DeAlBEA(Sn/Al = 0.15)上分散铂,当 Pt/Al = 0.001 时,产生的铂低聚物的平均 Pt-Pt 配位数为 3,但当 Pt/Al 比率为 0.013 时,形成的铂纳米粒子的 Pt-Pt 配位数为 9。Pt-Sn-DeAlBEA 对丙烯具有高选择性(97%)和高脱氢率。我们计算了正向速率常数,并将其与文献中报道的各种铂和铂硒催化剂的测定值进行了比较。本研究制备的 Pt-Sn-DeAlBEA 催化剂的正向速率常数明显高于之前报道的 Pt 和 PtSn 催化剂。对铂/铝比例不同但锡/铝比例相同的铂锡-DeAlBEA 催化剂进行了 PDH 动力学测量。在所有情况下,动力学均由 Langmuir-Hinshelwood 速率表达式描述,该表达式在丙烷中为一阶,并受到丙烷吸附的抑制。所有三种催化剂的丙烷吸附表观活化能和吸附焓的相似性表明,活性物种是与 DeAlBEA 框架紧密结合的极小 Pt3Sn 团簇。
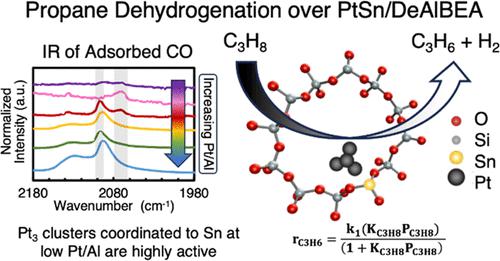
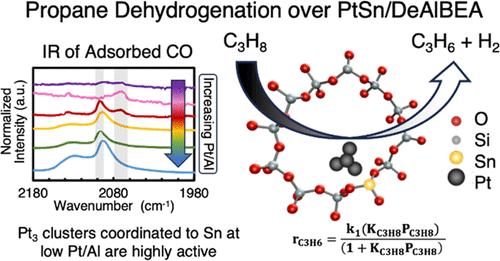
Recent research has found that dealuminated zeolite BEA (DeAlBEA) is an attractive support for the dispersion of Pt and PtSn species that serve as catalysts for propane dehydrogenation (PDH). In this study, we report the preparation, structural characterization, and PDH activities of Pt-Sn-DeAlBEA catalysts as a function of the Pt/Al ratio (here Al represents the amount of Al present in the parent zeolite H-BEA). The support Sn-DeAlBEA was prepared by introduction of Sn to DeAlBEA. Characterization of this material by X-ray absorption spectroscopy (XAS) and UV–vis spectroscopy revealed that the Sn incorporated into the BEA framework as Sn(IV) cations. Pt-Sn-DeAlBEA catalysts were prepared with Pt/Al ratios (0.001–0.026) and were characterized with infrared (IR) spectroscopy of adsorbed probe molecules and XAS to understand the effect of changing Pt loading on the structure of Pt in Pt-Sn-DeAlBEA. Pt dispersion on DeAlBEA (i.e., Pt-DeAlBEA) produced Pt nanoparticles with an average Pt–Pt coordination number of 9 (∼25 Å) for Pt/Al ratios of 0.001 and above. By contrast, dispersion of Pt on Sn-DeAlBEA (Sn/Al = 0.15) produced Pt oligomers with an average Pt–Pt coordination number of 3 for Pt/Al = 0.001, but for Pt/Al ratios >0.013, Pt nanoparticles formed with a Pt–Pt coordination number of 9. Pt-Sn-DeAlBEA exhibited high selectivity to propene (>97%) and high dehydrogenation rates. Forward rate constants were calculated and compared with values determined for various Pt and PtSn catalysts reported in the literature. The Pt-Sn-DeAlBEA catalysts prepared in this study exhibited significantly higher forward rate constants than those previously reported for Pt and PtSn catalysts. The kinetics of PDH were measured for Pt-Sn-DeAlBEA catalysts with different Pt/Al ratios but identical Sn/Al ratios. In all cases, the kinetics are described by a Langmuir–Hinshelwood rate expression, which is first order in propane and is inhibited by propane adsorption. The similarity of the apparent activation energies and enthalpies of propane adsorption for all three catalysts suggests that the active species are very small Pt3Sn clusters strongly bound to the framework of DeAlBEA.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


