比较基因组学揭示了食肉蝙蝠在脂质代谢方面的趋同适应性和低遗传多样性
IF 2.9
1区 生物学
Q1 Agricultural and Biological Sciences
引用次数: 0
摘要
饮食特化是研究动物适应性进化和保护生物学领域的一个重要因素。尽管蝙蝠在哺乳动物中表现出了无与伦比的食物多样性,但在这一群体中,专门的肉食仍然相对罕见。因此,我们对蝙蝠这种特殊食性的遗传和保护方面的理解在很大程度上仍然是未知的。为了研究食肉蝙蝠的分子适应性和保护遗传学,我们为食肉蝙蝠 Vampyrum spectrum 制作了一个新的基因组组装草案。此外,我们还利用该基因组与另一种亲缘关系较远的食肉蝙蝠 Megaderma lyra 进行了全基因组比较分析。我们的研究结果表明,与脂质代谢有关的基因在食肉蝙蝠的两个不同品系中表现出积极选择和趋同分子适应的特征。耐人寻味的是,我们发现肉食性蝙蝠饮食特化的进化伴随着作用于过氧化物酶体增殖激活受体通路基因的分子适应,而过氧化物酶体增殖激活受体是调节血浆脂质代谢和维持脂质平衡的关键。此外,我们的基因组分析还发现,这两种食肉蝙蝠的遗传多样性都很低。这种模式可归因于它们持续下降的种群规模和低水平的杂合性,这表明它们的脆弱性,并强调了保护工作的迫切需要。这些基因组学发现推进了我们对蝙蝠肉食性基因基础的理解,并强调了与肉食性蝙蝠物种相关的重大保护问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
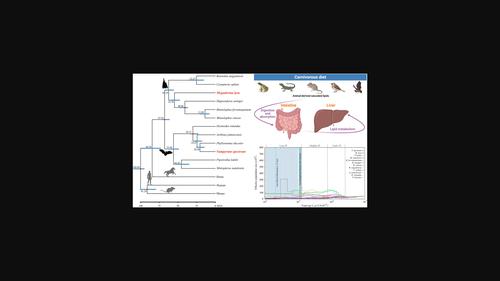
Comparative genomics reveals convergent adaptations in lipid metabolism and low genetic diversity in carnivorous bats
Dietary specialization stands as a major factor in the study of adaptive evolution and the field of conservation biology among animals. Although bats show unparalleled dietary diversification among mammals, specialized carnivory remains relatively rare within this group. Consequently, our comprehension of the genetic and conservation aspects associated with this specific dietary niche in bats has largely remained uncharted. To investigate molecular adaptations and conservation genetics in carnivorous bats, we produced a new draft genome assembly for the carnivorous bat Vampyrum spectrum. Furthermore, we utilized this genome alongside another distantly related carnivorous bat Megaderma lyra, to conduct genome-wide comparative analyses with other bat species. Our findings unveil that genes linked to lipid metabolism exhibit signatures of positive selection and convergent molecular adaptation in the two divergent lineages of carnivorous bats. Intriguingly, we have uncovered that the evolution of dietary specialization in carnivorous bats is accompanied by molecular adaptations acting on genes in the peroxisome proliferator-activated receptors pathways, which are crucial in regulating plasma lipid metabolism and sustaining lipid homeostasis. Additionally, our genomic analyses also reveal low genetic diversity in both carnivorous bat species. This pattern is attributed to their continuously declining population sizes and low levels of heterozygosity, signaling their vulnerability and emphasizing the pressing need for conservation efforts. These genomic discoveries advance our understanding of genetic underpinnings of carnivory in bats and underscore substantial conservation concerns associated with carnivorous bat species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Systematics and Evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
7.40
自引率
8.10%
发文量
1368
审稿时长
6-12 weeks
期刊介绍:
Journal of Systematics and Evolution (JSE, since 2008; formerly Acta Phytotaxonomica Sinica) is a plant-based international journal newly dedicated to the description and understanding of the biological diversity. It covers: description of new taxa, monographic revision, phylogenetics, molecular evolution and genome evolution, evolutionary developmental biology, evolutionary ecology, population biology, conservation biology, biogeography, paleobiology, evolutionary theories, and related subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


