重氮异构体与 OH 自由基反应的机理和动力学理论研究
IF 1.6
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
理论研究了吡嗪、哒嗪和嘧啶与羟基自由基的反应。用不同的电子结构方法得到的势垒高度表明,这些反应可以通过羟自由基抽取氢原子或羟自由基加到碳位点的方式竞争性地进行。然而,在 200 至 1500 K 的温度范围内计算得出的速率常数表明,隧道效应起了一定作用,从而导致在较低温度下有利于氢抽取通道的大分支比。本文章由计算机程序翻译,如有差异,请以英文原文为准。
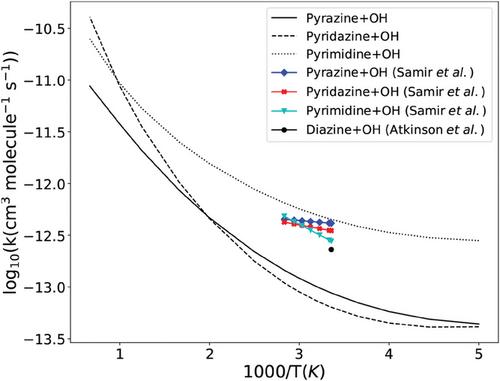
A theoretical study on the mechanism and kinetics of the reactions between diazine isomers and OH radicals
The reactions of pyrazine, pyridazine, and pyrimidine with hydroxyl radicals are theoretically studied. The barrier heights obtained with different electronic structure methods indicate that the reactions can competitively proceed via either abstraction of a hydrogen atom by an OH radical or OH addition to carbon sites. However, the rate constants computed within the temperature range 200 to 1500 K suggest that tunneling play a role resulting in large branching ratios in favor of hydrogen abstraction channels at lower temperatures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.30
自引率
6.70%
发文量
74
审稿时长
3 months
期刊介绍:
As the leading archival journal devoted exclusively to chemical kinetics, the International Journal of Chemical Kinetics publishes original research in gas phase, condensed phase, and polymer reaction kinetics, as well as biochemical and surface kinetics. The Journal seeks to be the primary archive for careful experimental measurements of reaction kinetics, in both simple and complex systems. The Journal also presents new developments in applied theoretical kinetics and publishes large kinetic models, and the algorithms and estimates used in these models. These include methods for handling the large reaction networks important in biochemistry, catalysis, and free radical chemistry. In addition, the Journal explores such topics as the quantitative relationships between molecular structure and chemical reactivity, organic/inorganic chemistry and reaction mechanisms, and the reactive chemistry at interfaces.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


