吡啶可调三铁烯基硼烷的电化学特性
IF 2.9
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
非水氧化还原液流电池(NARFB)为可持续的长期能源储存提供了前景广阔的解决方案。本文对三二茂铁硼烷(BFc3;Fc = 二茂铁,Cp2Fe,Cp = C5H5-)进行了研究,以了解其作为可调电解质用于 NARFB 的潜在作用。BFc3 含有三个容易氧化的二茂铁单元和一个具有空 p 轨道的中介硼烷,可根据亲核体的选择调整特定的氧化还原特性。因此,在一系列 4-R 取代的吡啶(R = NMe2、NH2、tBu、Et 或 I)和一系列氮供体强度存在的情况下,我们对 BFc3 的电化学行为(循环伏安法、充电/放电循环等)进行了研究。这些加合物产生了 E1/2 与对位取代常数 (σp) 的线性相关关系,仅根据供体的选择就显示出 200-300 mV 的可调谐性;此外还讨论了使用四氢呋喃、乙腈和二氯甲烷的溶剂效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
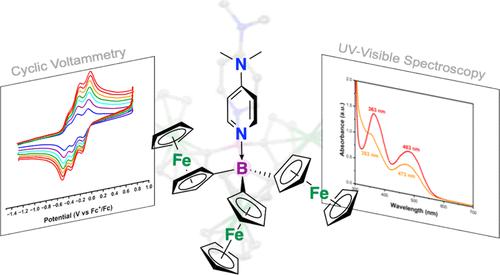
Electrochemical Properties of a Pyridine-Tunable Trisferrocenylborane
Non-aqueous redox flow batteries (NARFBs) offer promising solutions for sustainable and long-term energy storage. Herein, trisferrocenylborane (BFc3; Fc = ferrocene, Cp2Fe, and Cp = C5H5–) is examined for its potential role as a tunable anolyte for use in NARFBs. BFc3 contains three readily oxidized ferrocene units along with a mediating borane that hosts an empty p orbital, allowing tuned access to specific redox properties as a function of nucleophile choice. The electrochemical behavior (cyclic voltammetry, charging/discharging cycles, etc.) of BFc3 are accordingly examined in the presence of a series of 4-R-substituted pyridines (R = NMe2, NH2, tBu, Et, or I), with a range of nitrogen donor strengths. These adducts produce a linear correlation that relates E1/2 to the para-substituent constant (σp), showing a tunability of 200–300 mV based on donor choice alone; solvent effects using tetrahydrofuran, acetonitrile, and dichloromethane are additionally discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


