AMPA 受体的开放门形成一个 Ca2+ 结合位点,对调节离子转运至关重要
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
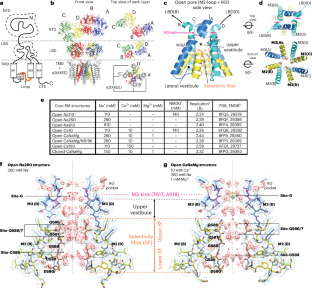
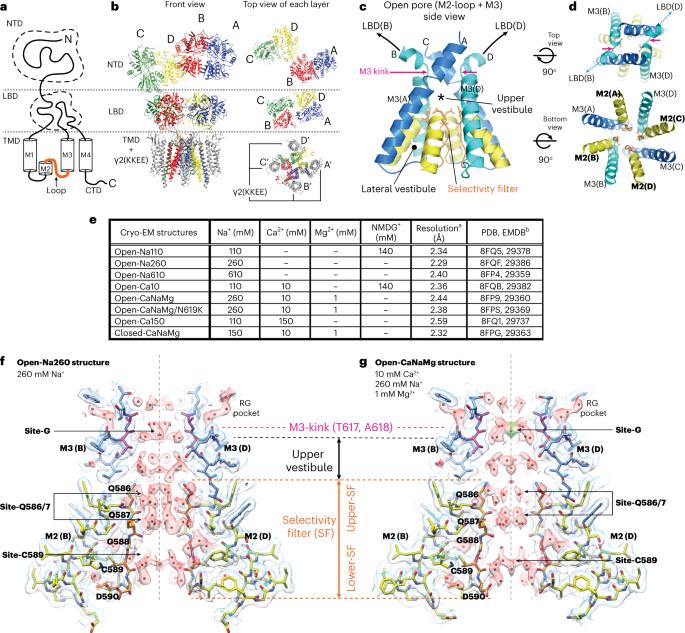
α-氨基-3-羟基-5-甲基-4-异恶唑-丙酸受体(AMPARs)是一种阳离子选择性离子通道,介导大脑中大多数快速兴奋性神经传递。尽管人们对其门控机制进行了广泛研究,但对阳离子如何穿过孔道的理解却一直难以捉摸。在这里,我们以 2.3-2.6 Å 的分辨率研究了 Ca2+ 可渗透 AMPARs(大鼠 GRIA2 flip-Q 异构体)开放孔中的推定离子和水密度。我们的研究表明,当通道闸门进入开放构型时,离子渗透途径会获得一个细胞外 Ca2+ 结合位点(位点-G)。位点-G 对 Ca2+ 而不是 Na+ 具有高度选择性,有利于 Ca2+ 进入孔的选择性过滤器。与癫痫发作相关的 N619K 突变与位点-G 相邻,可促进通道开放,但会减弱 Ca2+ 的结合,从而降低 Ca2+ 的通透性。我们的研究确定了位点-G的重要性,它与选择性滤过器的Q/R位点协调,确保Ca2+优先通过通道孔。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The open gate of the AMPA receptor forms a Ca2+ binding site critical in regulating ion transport
Alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) are cation-selective ion channels that mediate most fast excitatory neurotransmission in the brain. Although their gating mechanism has been studied extensively, understanding how cations traverse the pore has remained elusive. Here we investigated putative ion and water densities in the open pore of Ca2+-permeable AMPARs (rat GRIA2 flip-Q isoform) at 2.3–2.6 Å resolution. We show that the ion permeation pathway attains an extracellular Ca2+ binding site (site-G) when the channel gate moves into the open configuration. Site-G is highly selective for Ca2+ over Na+, favoring the movement of Ca2+ into the selectivity filter of the pore. Seizure-related N619K mutation, adjacent to site-G, promotes channel opening but attenuates Ca2+ binding and thus diminishes Ca2+ permeability. Our work identifies the importance of site-G, which coordinates with the Q/R site of the selectivity filter to ensure the preferential transport of Ca2+ through the channel pore. A calcium ion binding site, hidden in the highly conserved gate of the synaptic AMPA-type ionotropic glutamate receptor, reveals upon gating and controls ion transport across the membrane, providing new mechanistic insights on ion permeation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


