聚(2-烷氧基乙氧羰基亚甲基)的聚合后改性:聚合物主链中酮烯硅基缩醛重复单元的高效形成和反应活性
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
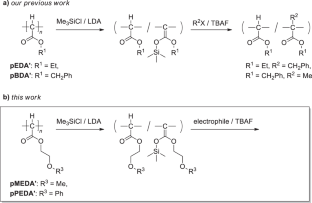
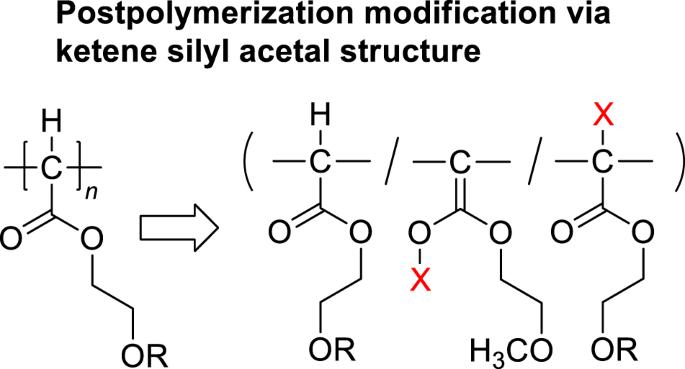
介绍了聚(2-甲氧基乙氧羰基亚甲基)(pMEDA')和聚(2-苯氧基乙氧羰基亚甲基)(pPEDA')的聚合后改性。这些聚合物与氯代三甲基硅烷(Me3SiCl)和二异丙基酰胺锂(LDA)的混合物反应,可有效地将烷氧羰基亚甲基重复单元转化为烯甲氧基硅基乙缩醛,从而得到后一种单元成分高达 93 摩尔%的产品。通过改变 Me3SiCl/LDA 与烷氧羰基亚甲基单元的进料比,可以控制产品中的乙缩酮硅烷成分。以四丁基氟化铵(TBAF)为介质,用溴化苄基对高度硅烷基化的聚合物进行苄基化反应,得到了含有侧链 O(主要)和主链 C(次要)苄基化单元以及未反应的酮烯硅基缩醛单元的聚合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Postpolymerization modification of poly(2-alkoxyethoxycarbonylmethylene)s: Efficient formation and reactivity of the ketene silyl acetal repeating units in the polymer main chain
Postpolymerization modifications of poly(2-methoxyethoxycarbonylmethylene) (pMEDA’) and poly(2-phenoxyethoxycarbonylmethylene) (pPEDA’) are described. The reactions of these polymers with mixtures of chlorotrimethylsilane (Me3SiCl) and lithium diisopropylamide (LDA) efficiently transformed the alkoxycarbonylmethylene repeating units to ketene silyl acetals to yield a product with up to 93 mol% composition of the latter unit. The ketene silyl acetal composition of the product was controlled by changing the feed ratio of Me3SiCl/LDA with respect to the alkoxycarbonylmethylene unit. Tetrabutylammonium fluoride (TBAF)-mediated benzylation of the highly silylated polymer with benzyl bromide yielded a polymer containing side chain O (major)- and main chain C (minor)-benzylated units along with the unreacted ketene silyl acetal unit. Postpolymerization modifications of poly(2-methoxyethoxycarbonylmethylene) and poly(2-phenoxyethoxycarbonylmethylene) with mixtures of Me3SiCl and LDA efficiently transformed the alkoxycarbonylmethylene repeating units to ketene silyl acetals to yield a product with up to 93 mol% composition of the latter unit. TBAF-mediated benzylation of the highly silylated polymer with benzyl bromide yielded a polymer containing side chain O (major)- and main chain C (minor)-benzylated units along with the unreacted ketene silyl acetal unit.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


