对 CAR T 细胞进行表观遗传学重编程,使其在体内持续发挥抗实体瘤的功能。
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
有限的 CAR T 细胞扩增和持久性阻碍了实体瘤患者的治疗反应。为了提高工程 T 细胞疗法的功能持久性,我们在人类 CAR T 细胞中对 SUV39H1 进行了基因干扰,SUV39H1 是组蛋白 3 赖氨酸 9 甲基转移酶,能促进异染色质的形成。这导致了表型 CAR-T 重编程,从而激发了最佳和持续的抗肿瘤功能。对肿瘤浸润CAR-T细胞进行的单细胞转录组(scRNA-seq)和染色质可及性(scATAC-seq)分析表明,CAR-T细胞早期重编程为自我更新的干样细胞群,所有亚群中功能障碍基因的表达均有所下降。此外,我们还提供了证据表明,SUV39H1 失活可在多次肿瘤再挑战后产生强大而持久的功能持续性。这为加强实体瘤的采纳细胞疗法开辟了一条安全之路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
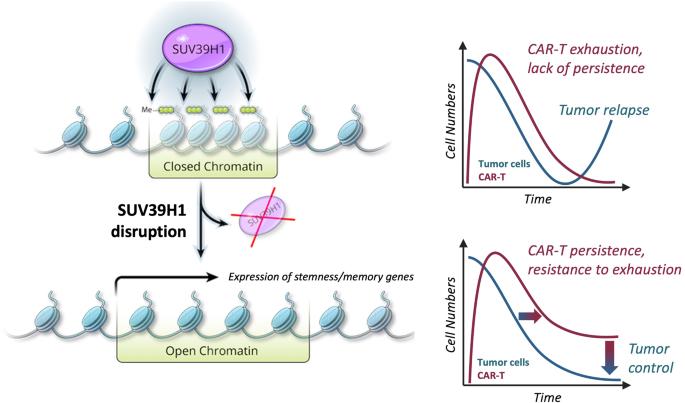
Epigenetic reprogramming of CAR T cells for in vivo functional persistence against solid tumors
Limited CAR T-cell expansion and persistence hinder therapeutic responses in solid cancer patients. To enhance the functional persistence of engineered T-cell therapies, we performed genetic disruption in human CAR T cells of SUV39H1, a histone 3 lysine 9 methyltransferase that promotes heterochromatin formation. This resulted in phenotypic CAR-T reprogramming that elicited optimal and sustained antitumor functionality. Single-cell transcriptomic (scRNA-seq) and chromatin accessibility (scATAC-seq) analyses of tumor-infiltrating CAR T cells showed early reprogramming into self-renewing, stem-like populations with decreased expression of dysfunction genes in all subpopulations. Moreover, we provided evidence that SUV39H1 inactivation elicits potent and durable functional persistence upon multiple tumor rechallenges. This opens a safe path to enhancing adoptive cell therapies for solid tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


