小分子介导控制超分子双特异性 T 细胞吸引剂的抗肿瘤活性和瘤外毒性。
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
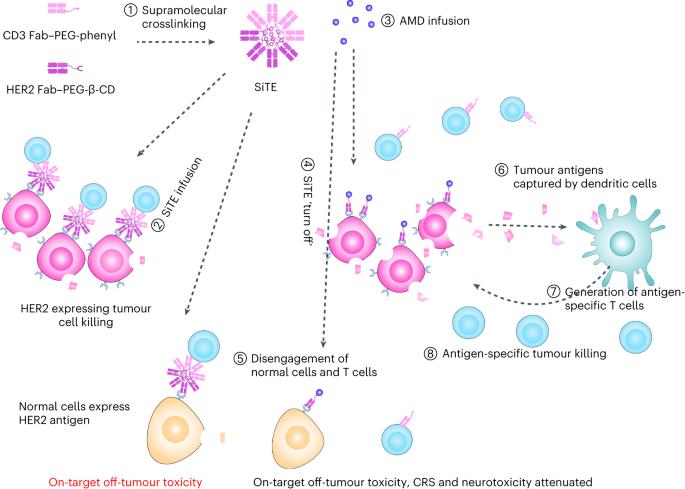
双特异性T细胞诱导剂诱导抗肿瘤毒性的更广泛临床应用受到其靶向瘤外毒性及相关神经毒性和细胞因子释放综合征的阻碍。在这里,我们展示了一种与乳腺肿瘤细胞上的 T 细胞共受体 CD3 和人表皮生长因子受体 2 结合的超分子双特异性 T 细胞吸引子的瘤外毒性,通过输注能分解超分子聚合体的小分子药物金刚烷胺,使 T 细胞与肿瘤细胞分离,从而阻止其瘤外毒性。在携带人类表皮生长因子受体 2 表达肿瘤并具有人类免疫系统的小鼠身上,大剂量静脉注射这种 "可切换 T 细胞纳米啮合剂 "可引起强烈的肿瘤特异性适应性免疫反应,从而防止肿瘤复发,同时金刚烷胺的输注限制了瘤外毒性、细胞因子释放综合征和神经毒性。可以进一步利用超分子化学来控制双特异性抗体的抗肿瘤活性和瘤外毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Small-molecule-mediated control of the anti-tumour activity and off-tumour toxicity of a supramolecular bispecific T cell engager
The broader clinical use of bispecific T cell engagers for inducing anti-tumour toxicity is hindered by their on-target off-tumour toxicity and the associated neurotoxicity and cytokine-release syndrome. Here we show that the off-tumour toxicity of a supramolecular bispecific T cell engager binding to the T cell co-receptor CD3 and to the human epidermal growth factor receptor 2 on breast tumour cells can be halted by disengaging the T cells from the tumour cells via the infusion of the small-molecule drug amantadine, which disassembles the supramolecular aggregate. In mice bearing human epidermal growth factor receptor 2-expressing tumours and with a human immune system, high intravenous doses of such a ‘switchable T cell nanoengager’ elicited strong tumour-specific adaptive immune responses that prevented tumour relapse, while the infusion of amantadine restricted off-tumour toxicity, cytokine-release syndrome and neurotoxicity. Supramolecular chemistry may be further leveraged to control the anti-tumour activity and off-tumour toxicity of bispecific antibodies. The off-tumour toxicity of a supramolecular bispecific T cell engager can be halted by disengaging T cells from the tumour cells via the disassembly of the supramolecular aggregate through the infusion of the small-molecule drug amantadine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


