调节性 T 细胞的调节和分化及其在自身免疫性疾病中的功能障碍。
IF 67.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
FOXP3+ 调节性 T(Treg)细胞是一种独特的细胞系,在调节免疫反应中发挥着核心作用,这一发现加深了人们对自身耐受性的理解。转录因子 FOXP3 在 Treg 细胞系的确定和维持中起着关键作用,但不足以充分发挥 Treg 细胞的抑制潜能,这表明 Treg 细胞功能的微调是由其他因素协调的。此外,最近有研究表明,与 FOXP3 无关的机制也会导致 Treg 细胞功能障碍。人类的 FOXP3 基因突变会导致致命的暴发性系统性自身炎症(IPEX 综合征)。然而,目前仍不清楚 Treg 细胞功能障碍在多大程度上导致了常见自身免疫性疾病的病理生理学。在这篇综述中,我们将讨论 Treg 细胞在外周的起源、诱导 Treg 细胞的多层机制,以及维持人类和小鼠 Treg 细胞抑制功能的 FOXP3 依赖性和 FOXP3 非依赖性细胞程序。此外,我们还研究了在多发性硬化症、炎症性肠病、系统性红斑狼疮和类风湿性关节炎等常见自身免疫性疾病中 Treg 细胞功能失调的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
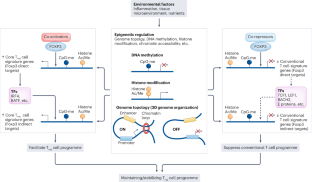
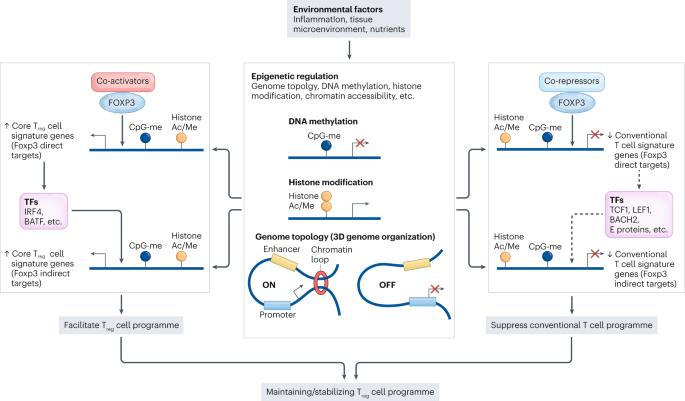
The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
The discovery of FOXP3+ regulatory T (Treg) cells as a distinct cell lineage with a central role in regulating immune responses provided a deeper understanding of self-tolerance. The transcription factor FOXP3 serves a key role in Treg cell lineage determination and maintenance, but is not sufficient to enable the full potential of Treg cell suppression, indicating that other factors orchestrate the fine-tuning of Treg cell function. Moreover, FOXP3-independent mechanisms have recently been shown to contribute to Treg cell dysfunction. FOXP3 mutations in humans cause lethal fulminant systemic autoinflammation (IPEX syndrome). However, it remains unclear to what degree Treg cell dysfunction is contributing to the pathophysiology of common autoimmune diseases. In this Review, we discuss the origins of Treg cells in the periphery and the multilayered mechanisms by which Treg cells are induced, as well as the FOXP3-dependent and FOXP3-independent cellular programmes that maintain the suppressive function of Treg cells in humans and mice. Further, we examine evidence for Treg cell dysfunction in the context of common autoimmune diseases such as multiple sclerosis, inflammatory bowel disease, systemic lupus erythematosus and rheumatoid arthritis. In this Review, the authors discuss the origins of regulatory T (Treg) cells in the periphery and the mechanisms by which Treg cells are induced, as well as the regulation of the suppressive function of these cells. Moreover, they examine evidence for and mechanisms of Treg cell dysfunction in common autoimmune diseases such as multiple sclerosis, inflammatory bowel disease, systemic lupus erythematosus and rheumatoid arthritis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Immunology
医学-免疫学
CiteScore
93.40
自引率
0.40%
发文量
131
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Immunology is a journal that provides comprehensive coverage of all areas of immunology, including fundamental mechanisms and applied aspects. It has two international standard serial numbers (ISSN): 1474-1733 for print and 1474-1741 for online. In addition to review articles, the journal also features recent developments and new primary papers in the field, as well as reflections on influential people, papers, and events in the development of immunology. The subjects covered by Nature Reviews Immunology include allergy and asthma, autoimmunity, antigen processing and presentation, apoptosis and cell death, chemokines and chemokine receptors, cytokines and cytokine receptors, development and function of cells of the immune system, haematopoiesis, infection and immunity, immunotherapy, innate immunity, mucosal immunology and the microbiota, regulation of the immune response, signalling in the immune system, transplantation, tumour immunology and immunotherapy, and vaccine development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


