炎症小体结构的机制启示。
IF 67.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
炎症小体是一种超分子复合物,它在细胞质中形成,对与病原体相关和与损伤相关的刺激以及其他扰乱细胞平衡的危险信号做出反应,导致宿主以细胞因子释放和程序性细胞死亡(热解)的形式做出防御反应。炎症小体的活动与许多人类疾病密切相关,包括罕见的自身炎症遗传综合征、心血管疾病、神经变性和癌症。近年来,人们发现了一系列炎症小体成分及其功能,增进了我们对整个机制的了解。在此,我们从结构和机理研究的角度回顾了炎性体生物学的最新进展。我们重点研究了典型炎症小体中研究最为深入的成分--NAIP-NLRC4、NLRP3、NLRP1、CARD8 和 caspase-1,以及非典型炎症小体中的 caspase-4、caspase-5 和 caspase-11,以及炎症小体效应物 GSDMD 和 NINJ1。这些结构研究揭示了炎性体如何组装和调控,以及它们如何诱导 IL-1 家族细胞因子的释放和诱导膜在高温分解过程中破裂的重要信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
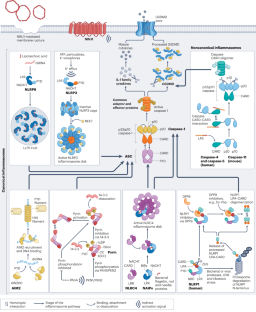
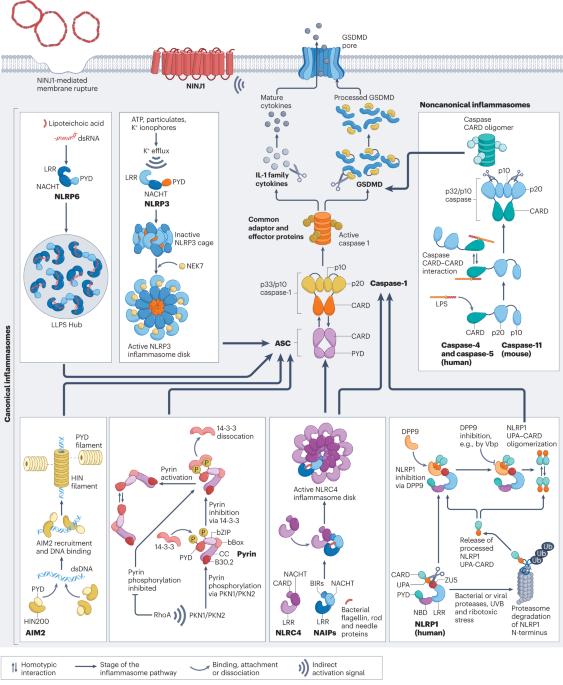
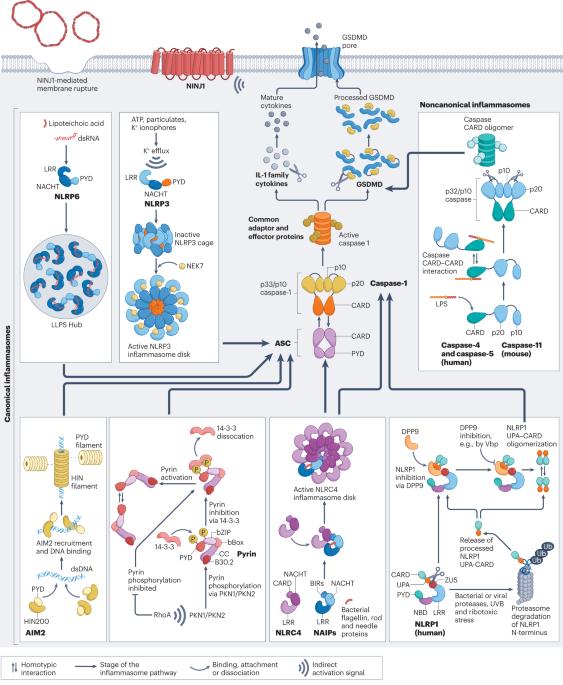
Mechanistic insights from inflammasome structures
Inflammasomes are supramolecular complexes that form in the cytosol in response to pathogen-associated and damage-associated stimuli, as well as other danger signals that perturb cellular homoeostasis, resulting in host defence responses in the form of cytokine release and programmed cell death (pyroptosis). Inflammasome activity is closely associated with numerous human disorders, including rare genetic syndromes of autoinflammation, cardiovascular diseases, neurodegeneration and cancer. In recent years, a range of inflammasome components and their functions have been discovered, contributing to our knowledge of the overall machinery. Here, we review the latest advances in inflammasome biology from the perspective of structural and mechanistic studies. We focus on the most well-studied components of the canonical inflammasome — NAIP–NLRC4, NLRP3, NLRP1, CARD8 and caspase-1 — as well as caspase-4, caspase-5 and caspase-11 of the noncanonical inflammasome, and the inflammasome effectors GSDMD and NINJ1. These structural studies reveal important insights into how inflammasomes are assembled and regulated, and how they elicit the release of IL-1 family cytokines and induce membrane rupture in pyroptosis. This Review highlights new insights into the biology of inflammasomes from the perspective of structural and mechanistic studies, revealing how the supramolecular complexes that activate inflammatory caspases are assembled and regulated, to induce cytokine maturation and release, as well as pyroptotic cell death.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Immunology
医学-免疫学
CiteScore
93.40
自引率
0.40%
发文量
131
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Immunology is a journal that provides comprehensive coverage of all areas of immunology, including fundamental mechanisms and applied aspects. It has two international standard serial numbers (ISSN): 1474-1733 for print and 1474-1741 for online. In addition to review articles, the journal also features recent developments and new primary papers in the field, as well as reflections on influential people, papers, and events in the development of immunology. The subjects covered by Nature Reviews Immunology include allergy and asthma, autoimmunity, antigen processing and presentation, apoptosis and cell death, chemokines and chemokine receptors, cytokines and cytokine receptors, development and function of cells of the immune system, haematopoiesis, infection and immunity, immunotherapy, innate immunity, mucosal immunology and the microbiota, regulation of the immune response, signalling in the immune system, transplantation, tumour immunology and immunotherapy, and vaccine development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


