通过高功率微波形成的活性物质会诱导 DNA 损伤,并促进肺癌细胞内在途径介导的凋亡:体外研究
IF 6.2
3区 综合性期刊
Q1 Multidisciplinary
引用次数: 0
摘要
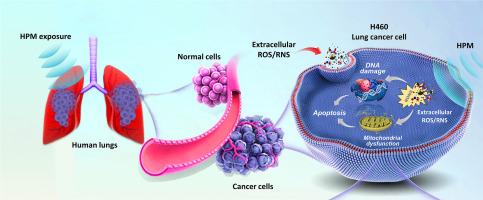
肺癌仍然是全球第二大最常见的癌症诊断和癌症相关死亡的主要原因,这需要新的和有效的治疗策略。在考虑治疗方案时,非小细胞肺癌(NSCLC)仍然是一个挑战,寻求新的治疗策略。高功率微波(HPM)的发展促进了新技术的进步以及对已经使用的技术的改进。HPM对NSCLC的影响尚未有研究。在这项工作中,我们首次揭示了脉冲HPM对NSCLC (H460和A549)的影响以及最可能的潜在机制。两个NSCLC (H460和A549)细胞和肺正常MRC5暴露于HPM(15、30、45和60)脉冲(2.1 mJ/脉冲)下。暴露后,在12、24、48和72小时观察到影响。HPM主要通过~ 27 kV/cm的强电场增加细胞内活性物质的水平,这改变了NSCLC的活力、线粒体活性和死亡率。提出了一种HPM产生胞内活性物质的模型。发现HPM通过DNA损伤(ATR/ATM、Chk1/Chk2和P53上调)和凋亡标记物表达增加影响NSCLC。NAC清除剂和cptio抑制剂证实了活性物质是细胞作用的主要原因。为了确保实际使用的适用性,蒙皮深度计算为30 mm。ROS在诱导细胞效应中起主要作用,NO可能起一定作用。这些发现阐明了hpm诱导细胞死亡的细胞机制,潜在地推进了治疗非小细胞肺癌的治疗方法,并为该领域的未来研究迈出了有用的第一步。此外,这项技术有可能作为癌症治疗非手术方法的辅助手段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formation of reactive species via high power microwave induced DNA damage and promoted intrinsic pathway-mediated apoptosis in lung cancer cells: An in vitro investigation
Lung cancer continues to be the second most common cancer diagnosed and the main cause of cancer-related death globally, which requires novel and effective treatment strategies. When considering treatment options, non-small cell lung cancer (NSCLC) remained a challenge, seeking new therapeutic strategies. High-power microwave (HPM) progressions have facilitated the advancement of new technologies as well as improvements to those already in use. The impact of HPM on NSCLC has not been investigated before. In this work, we uncovered the effect of pulsed HPM on NSCLC (H460 and A549) for the first time and the most likely underlying mechanisms. Two NSCLC (H460 and A549) cells and lung normal MRC5 were exposed to HPM (15, 30, 45, and 60) pulses (2.1 mJ/pulse). After exposure, the effects were observed at 12, 24, 48, and 72 h. HPM primarily increases the level of intracellular reactive species by a strong electric field of ∼27 kV/cm, which altered NSCLC viability, mitochondrial activity, and death rates. A model for the production of intracellular reactive species by HPM was also presented. NSCLC is found to be affected by HPM through DNA damage (upregulation of ATR/ATM, Chk1/Chk2, and P53) and increased expression of apoptotic markers. NAC scavenger and CPTIO-inhibitor confirm that the reactive species are mainly accountable for cellular effects. In order to ensure suitability for real-world usage, the skin depth was calculated as 30 mm. ROS played a main role in inducing cellular effects, with NO species possibly playing a contributing role. These findings clarify the cellular mechanisms underlying HPM-induced cell death, potentially advancing therapeutic approaches for treating NSCLC, and a useful first step for future investigations in this area. Moreover, this technique has the potential to serve as an adjunct to non-surgical methods in cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fundamental Research
Multidisciplinary-Multidisciplinary
CiteScore
4.00
自引率
1.60%
发文量
294
审稿时长
79 days
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


