苄基 C(sp3)-H 氮化:铜催化与铁催化
IF 1.5
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过氢原子抽取(HAT)生成苄基自由基是近年来的研究重点,各种 C(sp3)-H 键官能化方案都是基于这一基本步骤开发的。我们在此报告铜催化和铁催化的 C(sp3)-H 苄基叠氮反应,分别使用 mCPBA 和 NFSI 作为氧化剂,TMSN3 作为叠氮源。该反应被认为是通过原位生成的以杂原子为中心的自由基在分子间抽取苄基氢而引发的。Fe(OTf)3 催化的叠氮化反应具有良好的化学选择性,因为它优先发生在二级和三级苄基 C(sp3)-H 键上,而不是一级苄基和三级脂肪族碳上。此外,还记录了开发催化对映体选择性工艺的努力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
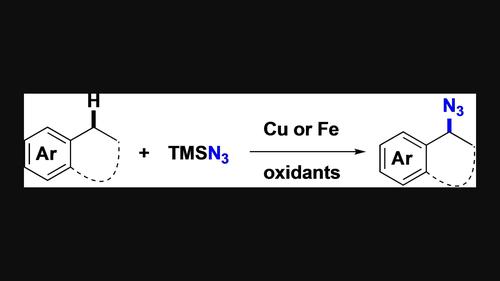
Benzylic C(sp3)‐H Azidation: Copper vs Iron Catalysis
The generation of benzylic radicals through hydrogen atom abstraction (HAT) has been a recent research focus and various C(sp3)‐H bond functionalization protocols have been developed relying on this elementary step. We report herein copper‐ and iron‐catalyzed C(sp3)‐H benzylic azidation reactions using mCPBA and NFSI as oxidant, respectively, and TMSN3 as azide source. The reaction is thought to be initiated via intermolecular abstraction of benzylic hydrogen by the in situ generated heteroatom‐centered radicals. The Fe(OTf)3‐catalyzed azidation protocol displays good chemoselectivity as it takes place preferentially at the secondary and tertiary benzylic C(sp3)‐H bonds over the primary benzylic and tertiary aliphatic carbons. Efforts on the development of catalytic enantioselective processes are also documented.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Helvetica Chimica Acta
化学-化学综合
CiteScore
3.00
自引率
0.00%
发文量
60
审稿时长
2.3 months
期刊介绍:
Helvetica Chimica Acta, founded by the Swiss Chemical Society in 1917, is a monthly multidisciplinary journal dedicated to the dissemination of knowledge in all disciplines of chemistry (organic, inorganic, physical, technical, theoretical and analytical chemistry) as well as research at the interface with other sciences, where molecular aspects are key to the findings. Helvetica Chimica Acta is committed to the publication of original, high quality papers at the frontier of scientific research. All contributions will be peer reviewed with the highest possible standards and published within 3 months of receipt, with no restriction on the length of the papers and in full color.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


