CAMTA3 转录因子中的天然单核苷酸多态性可调节其功能及其靶基因的转录
IF 4.8
1区 农林科学
Q1 PLANT SCIENCES
引用次数: 0
摘要
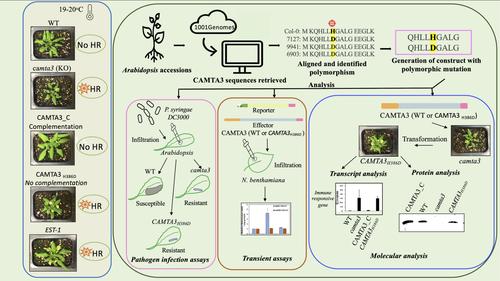
CAMTA3是一种Ca2+/钙调蛋白结合型转录因子,是拟南芥植物免疫的关键调控因子。在这里,我们发现了一种新的自然发生的单核苷酸多态性,这种多态性会在许多拟南芥生态型中导致错义非保守突变(CAMTA3H386D)。CAMTA3 的这一区域不属于任何先前表征的调控域。为了研究这一变化对 CAMTA3 功能的影响,我们将 CAMTA3H386D 引入了 camta3,这是一种功能缺失突变体,表现出组成型细胞死亡表型、叶片萎黄病变和植株矮小。对这些品系的表型和分子分析表明,在 camta3 突变体中表达 CAMTA3H386D 并不能补充突变体的表型。此外,含有 CAMTA3H386D 的生态型也表现出 camta3 的表型。与 camta3 一样,在 CAMTA3H386D 株系和拟南芥 7127(Est-1)和 9941(Fei-0)中,与水杨酸生物合成和病原体反应相关的标记基因上调,这表明 H386D 突变改变了 CAMTA3 在调节已知靶基因表达方面的活性。在烟曲霉瞬时表达试验中,CAMTA3H386D 未能诱导由快速胁迫反应元件(RSRE)驱动的荧光素酶报告基因的表达,而该元件含有 CAMTA3 的已知结合位点,这表明 CAMTA3H386D 突变削弱了其激活靶基因的能力。表达 CAMTA3H386D 的转基因品系和经过测试的天然品种显示出更高的 H2O2 水平,并增强了对细菌病原体 Pseudomonas syringae pv. tomato DC3000 的抗性。总之,我们的研究结果表明,CAMTA3 先前未知调控区中的 H386D 突变对其功能至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A natural single-nucleotide polymorphism in the CAMTA3 transcription factor regulates its function and transcription of its target genes
CAMTA3, a Ca2+/calmodulin-binding transcription factor, is a key regulator of plant immunity in Arabidopsis. Here, we identified a novel naturally occurring single-nucleotide polymorphism that results in a missense nonconservative mutation (CAMTA3H386D) in many Arabidopsis ecotypes. This region of CAMTA3 is not part of any previously characterized regulatory domains. To study the consequence of this change on the function of CAMTA3, we introduced the CAMTA3H386D into camta3, a loss-of-function mutant that exhibits a constitutive cell death phenotype, chlorotic lesions on leaves, and reduced plant size. Phenotypic and molecular analysis of these lines indicated that the expression of CAMTA3H386D in the camta3 mutant did not complement the mutant phenotypes. Also, the ecotypes containing the CAMTA3H386D exhibited camta3 phenotypes. Marker genes associated with salicylic acid biosynthesis and pathogen response were upregulated in the CAMTA3H386D lines and the Arabidopsis accessions 7127 (Est-1) and 9941 (Fei-0), as in camta3, indicating that H386D mutation alters CAMTA3 activity in regulating the expression of known target genes. In Nicotiana benthamiana transient expression assays, CAMTA3H386D failed to induce the expression of a luciferase reporter gene driven by the rapid stress-responsive elements (RSRE) that contain the known binding sites of CAMTA3, suggesting that CAMTA3H386D mutation impairs its ability to activate its target genes. Transgenic lines and tested natural accessions expressing CAMTA3H386D showed enhanced levels of H2O2 and increased resistance to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000. Collectively, our results indicate that the H386D mutation in a previously unknown regulatory region of CAMTA3 is essential for its function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular plant pathology
生物-植物科学
CiteScore
9.40
自引率
4.10%
发文量
120
审稿时长
6-12 weeks
期刊介绍:
Molecular Plant Pathology is now an open access journal. Authors pay an article processing charge to publish in the journal and all articles will be freely available to anyone. BSPP members will be granted a 20% discount on article charges. The Editorial focus and policy of the journal has not be changed and the editorial team will continue to apply the same rigorous standards of peer review and acceptance criteria.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


