对纤毛在细胞信号传导中功能的新机理认识
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
原生纤毛是存在于人体大多数细胞上的单生、不动的感觉细胞器,广泛参与人体健康、生理和疾病的过程。纤毛为信号转导创造了独特的环境,在一个相对较小的空间内严格控制蛋白质、脂质和第二信使的浓度,从而实现生物信息的接收、传输和整合。在本综述中,我们将以纤毛 G 蛋白偶联受体(GPCR)、刺猬(Hh)通路和多细胞蛋白离子通道这三种信号通路为例,讨论纤毛如何在细胞-细胞通信中发挥信号枢纽的作用。我们回顾了这些纤毛信号通路的缺陷如何导致一组被称为 "纤毛疾病 "的异质性病症,包括代谢综合征、出生缺陷和多囊肾。对这些途径转导机制的新认识揭示了这些基于纤毛的信号途径之间的共同主题,这些主题可能也适用于其他途径。这些机理研究揭示了纤毛如何在整个人类生物学中协调正常和病理生理信号输出。本文章由计算机程序翻译,如有差异,请以英文原文为准。
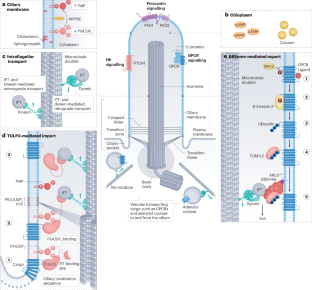
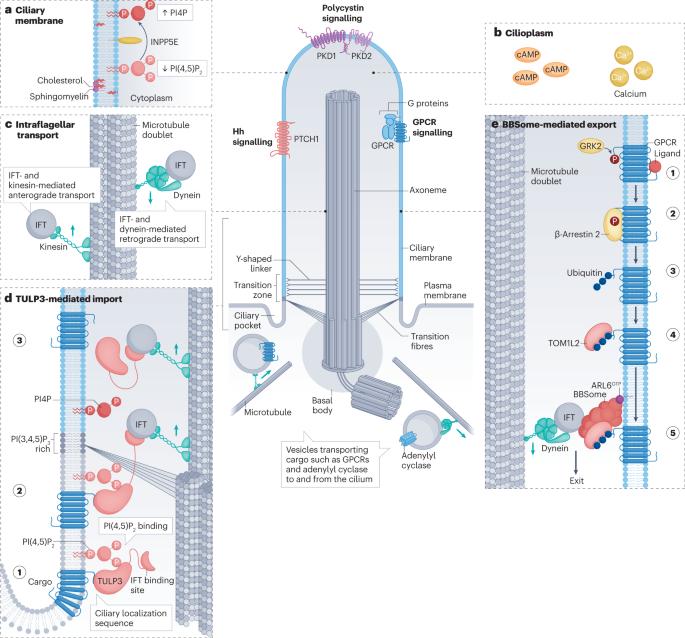
Emerging mechanistic understanding of cilia function in cellular signalling
Primary cilia are solitary, immotile sensory organelles present on most cells in the body that participate broadly in human health, physiology and disease. Cilia generate a unique environment for signal transduction with tight control of protein, lipid and second messenger concentrations within a relatively small compartment, enabling reception, transmission and integration of biological information. In this Review, we discuss how cilia function as signalling hubs in cell–cell communication using three signalling pathways as examples: ciliary G-protein-coupled receptors (GPCRs), the Hedgehog (Hh) pathway and polycystin ion channels. We review how defects in these ciliary signalling pathways lead to a heterogeneous group of conditions known as ‘ciliopathies’, including metabolic syndromes, birth defects and polycystic kidney disease. Emerging understanding of these pathways’ transduction mechanisms reveals common themes between these cilia-based signalling pathways that may apply to other pathways as well. These mechanistic insights reveal how cilia orchestrate normal and pathophysiological signalling outputs broadly throughout human biology. Cilia are microtubule-based cell projections that provide a unique environment with precise protein, lipid and second messenger concentrations, thereby creating specialized signalling hubs. This Review discusses recent multidisciplinary, mechanistic insights into cilia-based signalling pathways during development and homeostasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


