间质性膀胱炎/膀胱疼痛综合征的氧化应激机制。
IF 12.1
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
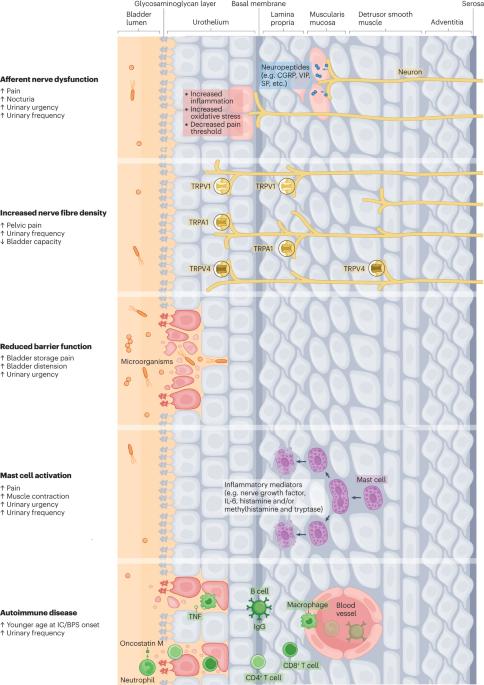
间质性膀胱炎/膀胱疼痛综合征(IC/BPS)的特征是膀胱和/或盆腔疼痛、尿急、尿频和夜尿增多。目前对间质性膀胱炎/膀胱疼痛综合征的病理生理学尚不十分清楚,其理论包括慢性炎症、自身免疫失调、细菌性膀胱炎、尿道功能障碍、糖胺聚糖(GAG)屏障缺乏和尿液细胞毒性。目前有多种治疗方案,包括行为干预、口服药物、膀胱内灌注和尿液扩张术等;然而,许多临床试验都以失败告终,患者的治疗效果也不尽如人意,这很可能是由于 IC/BPS 表型的异质性和非针对性干预措施的使用所致。氧化应激与 IC/BPS 的发病机制有关,因为活性氧通过参与多种分子机制损害膀胱功能。激酶信号通路、痛觉受体、肥大细胞激活、尿道失调和昼夜节律紊乱都与活性氧和 IC/BPS 有关。然而,要全面揭示氧化应激在 IC/BPS 发病机制中的作用,还需要进一步的研究。开发可操控这些通路的新模型将有助于这项研究,并能进一步调查有前景的治疗目标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanisms of oxidative stress in interstitial cystitis/bladder pain syndrome
Interstitial cystitis/bladder pain syndrome (IC/BPS) is characterized by bladder and/or pelvic pain, increased urinary urgency and frequency and nocturia. The pathophysiology of IC/BPS is poorly understood, and theories include chronic inflammation, autoimmune dysregulation, bacterial cystitis, urothelial dysfunction, deficiency of the glycosaminoglycan (GAG) barrier and urine cytotoxicity. Multiple treatment options exist, including behavioural interventions, oral medications, intravesical instillations and procedures such as hydrodistension; however, many clinical trials fail, and patients experience an unsatisfactory treatment response, likely owing to IC/BPS phenotype heterogeneity and the use of non-targeted interventions. Oxidative stress is implicated in the pathogenesis of IC/BPS as reactive oxygen species impair bladder function via their involvement in multiple molecular mechanisms. Kinase signalling pathways, nociceptive receptors, mast-cell activation, urothelial dysregulation and circadian rhythm disturbance have all been linked to reactive oxygen species and IC/BPS. However, further research is necessary to fully uncover the role of oxidative stress in the pathways driving IC/BPS pathogenesis. The development of new models in which these pathways can be manipulated will aid this research and enable further investigation of promising therapeutic targets. Oxidative stress and reactive oxygen species contribute to the pathophysiology of interstitial cystitis/bladder pain syndrome through their effects on molecular pathways. Here, the authors discuss these pathways, animal models of interstitial cystitis/bladder pain syndrome and treatment options for the disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Urology
医学-泌尿学与肾脏学
CiteScore
12.50
自引率
2.60%
发文量
123
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Urology is part of the Nature Reviews portfolio of journals.Nature Reviews' basic, translational and clinical content is written by internationally renowned basic and clinical academics and researchers. This journal targeted readers in the biological and medical sciences, from the postgraduate level upwards, aiming to be accessible to professionals in any biological or medical discipline.
The journal features authoritative In-depth Reviews providing up-to-date information on topics within a field's history and development. Perspectives, News & Views articles, and the Research Highlights section offer topical discussions and opinions, filtering primary research from various medical journals.
Covering a wide range of subjects, including andrology, urologic oncology, and imaging, Nature Reviews provides valuable insights for practitioners, researchers, and academics within urology and related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


