锂-锰-欧-氧体系中的相平衡
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
摘要 首次研究了温度范围为 700-1000°С 的锂-锰-铕-O 体系中的相平衡,并构建了氧分压为 21 kPa 时锂-锰-铕三角形内的浓度图。结果表明,与 LiEuO2-LiMnO2 和 LiEuO2-LiMn2O4 部分不同,LiEuO2-Li2MnO3 部分可表示为准二元。据测定,尖晶石 LiMn2O4(Fd \(\bar {3}\) m)中 Eu 对 Mn 的同构取代不超过 2 mol %,而 Li2MnO3(C2/m)则失去了单相特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
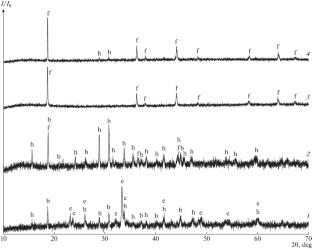
Phase Equilibria in the Li–Mn–Eu–O System
Phase equilibria in the Li–Mn–Eu–O system were studied for the first time in the temperature range 700–1000°С, and a concentration diagram was constructed within the Li–Mn–Eu triangle at an oxygen partial pressure of 21 kPa. It was shown that the LiEuO2–Li2MnO3 section can be represented as quasi-binary, unlike the sections LiEuO2–LiMnO2 and LiEuO2–LiMn2O4. It was determined that the isomorphic substitution of Eu for Mn in spinel LiMn2O4 (Fd\(\bar {3}\)m) does not exceed 2 mol %, while Li2MnO3 (C2/m) loses its single-phase character.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Physical Chemistry
化学-物理化学
CiteScore
1.50
自引率
0.00%
发文量
9
审稿时长
6-12 weeks
期刊介绍:
Doklady Physical Chemistry is a monthly journal containing English translations of current Russian research in physical chemistry from the Physical Chemistry sections of the Doklady Akademii Nauk (Proceedings of the Russian Academy of Sciences). The journal publishes the most significant new research in physical chemistry being done in Russia, thus ensuring its scientific priority. Doklady Physical Chemistry presents short preliminary accounts of the application of the state-of-the-art physical chemistry ideas and methods to the study of organic and inorganic compounds and macromolecules; polymeric, inorganic and composite materials as well as corresponding processes. The journal is intended for scientists in all fields of chemistry and in interdisciplinary sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


