T 细胞特异性适配蛋白与 Src 和 Tec 家族激酶的相互作用
IF 4.1
4区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
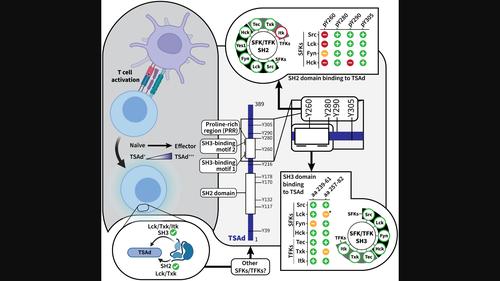
适配蛋白是细胞信号的灵活动态调节剂,对免疫细胞的功能非常重要。其中一种是 T 细胞特异性适配蛋白(TSAd),它能与 Src 家族激酶(SFKs)的非受体酪氨酸激酶 Src 和 Lck 以及 Tec 家族激酶(TFKs)的 Itk 相互作用。TSAd C末端的三个酪氨酸残基被Lck磷酸化,成为Src和Lck的Src同源2(SH2)结构域的对接位点。TSAd 富脯氨酸区(PRR)与 Lck、Src 和 Itk 中的 Src 同源 3(SH3)结构域结合。尽管已知有相互作用者,但 TSAd 在细胞信号传导中的作用在很大程度上仍不为人所知。TSAd 同时与 SFKs 和 TFKs 结合的能力可能表明它具有作为这两种激酶家族的通用支架的功能。利用 GST-pulldown 和肽阵列实验,我们发现 SFKs Fyn 和 Hck 以及 TFKs Tec 和 Txk 的 SH2 和 SH3 结构域都与 TSAd 相互作用。这与Itk形成了鲜明对比,后者仅通过其SH3结构域与TSAd相互作用。尽管我们的分析表明 TSAd 与 Fyn 既共表达又相互作用,但通过 Western 印迹和亲和纯化质谱分析,我们无法从 Jurkat 细胞中检测到 Fyn 与 TSAd 的共沉淀。这可能表明,TSAd-Fyn 在完整细胞中的相互作用可能受到其他因素的限制,如两种分子的亚细胞定位或竞争性结合伙伴的共同表达。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interaction of T-cell-specific adapter protein with Src- and Tec-family kinases
Adapter proteins are flexible and dynamic modulators of cellular signalling that are important for immune cell function. One of these, the T-cell-specific adapter protein (TSAd), interacts with the non-receptor tyrosine kinases Src and Lck of the Src family kinases (SFKs) and Itk of the Tec family kinases (TFKs). Three tyrosine residues in the TSAd C-terminus are phosphorylated by Lck and serve as docking sites for the Src homology 2 (SH2) domains of Src and Lck. The TSAd proline-rich region (PRR) binds to the Src homology 3 (SH3) domains found in Lck, Src and Itk. Despite known interactors, the role TSAd plays in cellular signalling remains largely unknown. TSAd's ability to bind both SFKs and TFKs may point to its function as a general scaffold for both kinase families. Using GST-pulldown as well as peptide array experiments, we found that both the SH2 and SH3 domains of the SFKs Fyn and Hck, as well as the TFKs Tec and Txk, interact with TSAd. This contrasts with Itk, which interacts with TSAd only through its SH3 domain. Although our analysis showed that TSAd is both co-expressed and may interact with Fyn, we were unable to co-precipitate Fyn with TSAd from Jurkat cells, as detected by Western blotting and affinity purification mass spectrometry. This may suggest that TSAd-Fyn interaction in intact cells may be limited by other factors, such as the subcellular localization of the two molecules or the co-expression of competing binding partners.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
5.40%
发文量
109
审稿时长
1 months
期刊介绍:
This peer-reviewed international journal publishes original articles and reviews on all aspects of basic, translational and clinical immunology. The journal aims to provide high quality service to authors, and high quality articles for readers.
The journal accepts for publication material from investigators all over the world, which makes a significant contribution to basic, translational and clinical immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


