听力损失与慢性肾病之间的多方面联系
IF 28.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
听力损失影响着近 16 亿人,是全球第三大致残原因。慢性肾脏病(CKD)也是一种常见疾病,与不良的临床结果和高昂的医疗费用有关。从发育的角度来看,负责听力的结构与肾脏有着共同的形态发生起源,导致家族性听力损失的基因异常也可能导致肾脏疾病。在细胞层面上,肾脏和耳蜗的正常功能都依赖于纤毛在顶端表面的活动,肾小管细胞和内耳的感觉上皮细胞使用类似的运输机制来改变管腔液体。这两个器官还具有相同的胶原蛋白 IV 基底膜网络。因此,听力和肾功能之间存在着密切的发育和生理联系。流行病学数据证明,慢性肾功能衰竭与感音神经性听力损失的分级和独立超额风险有关,这为这些理论考虑提供了支持。除了肾脏和耳蜗功能之间的发育和生理联系外,慢性肾功能衰竭患者的听力损失可能是由特定药物或治疗(包括血液透析)引起的。这两种常见疾病之间的关联并未得到普遍重视,但却对研究和临床实践具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
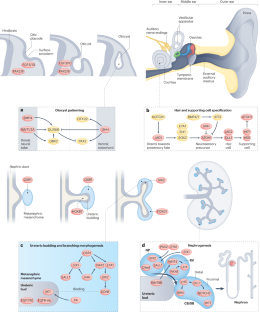
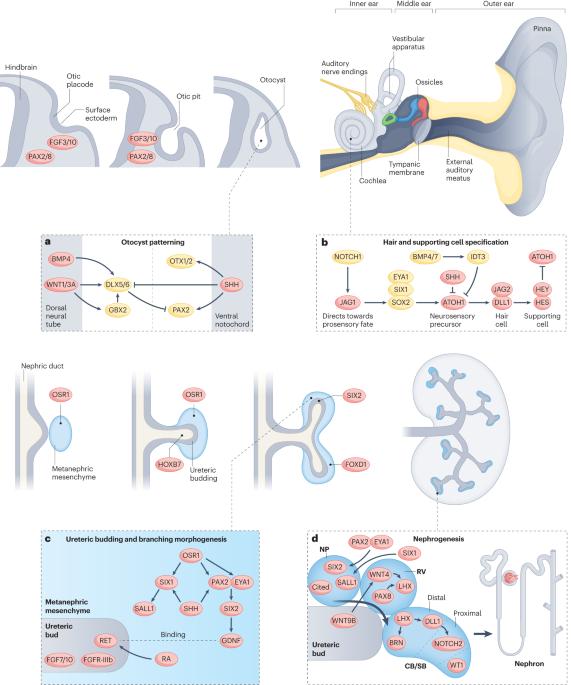
The multifaceted links between hearing loss and chronic kidney disease
Hearing loss affects nearly 1.6 billion people and is the third-leading cause of disability worldwide. Chronic kidney disease (CKD) is also a common condition that is associated with adverse clinical outcomes and high health-care costs. From a developmental perspective, the structures responsible for hearing have a common morphogenetic origin with the kidney, and genetic abnormalities that cause familial forms of hearing loss can also lead to kidney disease. On a cellular level, normal kidney and cochlea function both depend on cilial activities at the apical surface, and kidney tubular cells and sensory epithelial cells of the inner ear use similar transport mechanisms to modify luminal fluid. The two organs also share the same collagen IV basement membrane network. Thus, strong developmental and physiological links exist between hearing and kidney function. These theoretical considerations are supported by epidemiological data demonstrating that CKD is associated with a graded and independent excess risk of sensorineural hearing loss. In addition to developmental and physiological links between kidney and cochlear function, hearing loss in patients with CKD may be driven by specific medications or treatments, including haemodialysis. The associations between these two common conditions are not commonly appreciated, yet have important implications for research and clinical practice. Chronic kidney disease is associated with a graded and independent excess risk of sensorineural hearing loss. This Review describes how disruption of shared signalling pathways that are important for the development of both the ear and the kidney and/or the presence of clinical drivers, such as specific medications or treatments, may underlie these associations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


