全面了解缺铁性贫血导致的肠道微生物组和肠道屏障的改变。
IF 4.1
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:缺铁是导致贫血的首要原因,其治疗已被证明会恶化肠道健康。然而,迄今为止,尚未对缺铁性贫血期间的肠道屏障和肠道微生物组进行全面分析。本研究旨在进一步深入分析这两个方面,这将意味着向前迈进了一步,最大限度地减少铁补充剂对肠道健康的负面影响:方法:在动物模型中通过实验诱发 IDA。方法:在动物模型中实验性地诱导 IDA,使用霰弹枪测序分析结肠区域的肠道微生物组,同时通过组织学分析、mRNA 测序(RNA-Seq)、qPCR 和免疫荧光研究肠道屏障。还测定了脂多糖(LPS)和细菌特异性免疫球蛋白,以评估微生物的转移情况:结果:IDA期间,结肠中的微生物代谢转向增加某些氨基酸、短链脂肪酸和核苷酸的产生,梭状芽孢杆菌的种类增多。组织学分析显示结肠上皮细胞的结构发生了改变。RNA-Seq显示细胞外基质相关基因和蛋白质下调,上皮总体发育不良。血清 LPS 水平的升高以及针对菌群失调细菌的免疫反应的增强,都表明 IDA 期间肠道屏障的完整性受到了损害:IDA对肠道微生物组和肠道屏障产生了负面影响,引发了微生物转运的增加。这项研究强调了 IDA 期间肠道健康的恶化,以及在治疗该疾病时应解决的问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
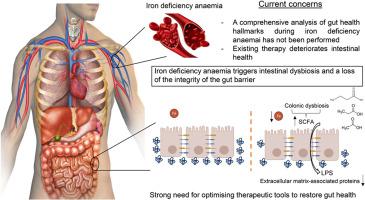
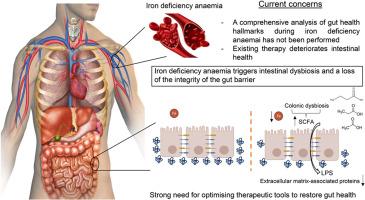
Comprehensive insight into the alterations in the gut microbiome and the intestinal barrier as a consequence of iron deficiency anaemia
Background
Iron deficiency is the top leading cause of anaemia, whose treatment has been shown to deteriorate gut health. However, a comprehensive analysis of the intestinal barrier and the gut microbiome during iron deficiency anemia (IDA) has not been performed to date. This study aims to delve further into the analysis of these two aspects, which will mean a step forward minimising the negative impact of iron supplements on intestinal health.
Methods
IDA was experimentally induced in an animal model. Shotgun sequencing was used to analyse the gut microbiome in the colonic region, while the intestinal barrier was studied through histological analyses, mRNA sequencing (RNA-Seq), qPCR and immunofluorescence assays. Determinations of lipopolysaccharide (LPS) and bacteria-specific immunoglobulins were performed to assess microbial translocation.
Results
Microbial metabolism in the colon shifted towards an increased production of certain amino acids, short chain fatty acids and nucleotides, with Clostridium species being enriched during IDA. Structural alterations of the colonic epithelium were shown by histological analysis. RNA-Seq revealed a downregulation of extracellular matrix-associated genes and proteins and an overall underdeveloped epithelium. Increased levels of serum LPS and an increased immune response against dysbiotic bacteria support an impairment in the integrity of the gut barrier during IDA.
Conclusions
IDA negatively impacts the gut microbiome and the intestinal barrier, triggering an increased microbial translocation. This study emphasizes the deterioration of gut health during IDA and the fact that it should be addressed when treating the disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomedical Journal
Medicine-General Medicine
CiteScore
11.60
自引率
1.80%
发文量
128
审稿时长
42 days
期刊介绍:
Biomedical Journal publishes 6 peer-reviewed issues per year in all fields of clinical and biomedical sciences for an internationally diverse authorship. Unlike most open access journals, which are free to readers but not authors, Biomedical Journal does not charge for subscription, submission, processing or publication of manuscripts, nor for color reproduction of photographs.
Clinical studies, accounts of clinical trials, biomarker studies, and characterization of human pathogens are within the scope of the journal, as well as basic studies in model species such as Escherichia coli, Caenorhabditis elegans, Drosophila melanogaster, and Mus musculus revealing the function of molecules, cells, and tissues relevant for human health. However, articles on other species can be published if they contribute to our understanding of basic mechanisms of biology.
A highly-cited international editorial board assures timely publication of manuscripts. Reviews on recent progress in biomedical sciences are commissioned by the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


