掺杂钾离子的纳米氧化锰卷增强了锌离子水电池的性能
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
α-二氧化锰是一种潜在的锌离子水电池正极材料,但其储锌电化学性能有待进一步提高。本文采用一步水热法合成了具有氧空位的钾离子掺杂二氧化锰纳米卷(K-MnO2)。结果表明,在 0.2 C 的电流密度下,其电化学比容量为 250.9 mAh/g,优于现有的商用 α-MnO2。在 1 C 的高电流下,这些电池的循环稳定性也有所提高。同步辐射和其他实验以及 DFT 理论计算提供了更多证据,证明掺 K 能有效调节 MnO2 中的金属键类型以及与氧原子共价键的平均电荷调节。当存在 Mn-O 键和 Mn-K 键时,K-MnO2 对 Zn2+ 的吸附作用突出,并进一步增强了 Zn2+ 的嵌入过程。同时,掺杂造成的氧缺陷促进了纳米卷轴结构的发展,从而增加了可用于电化学反应的活性位点,进而提高了 α-MnO2 的导电性。这项研究展示了通过引入钾掺杂策略优化锰基材料的潜力,从而提高水生锌离子电池的性能,并为相关研究提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
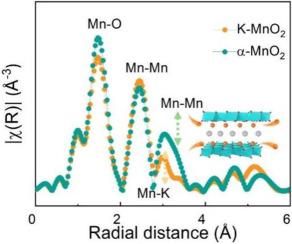
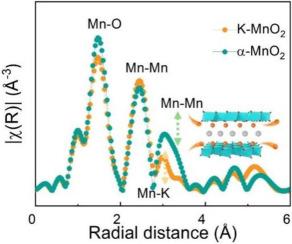
Potassium ion doped manganese oxide nanoscrolls enhanced the performance of aqueous zinc-ion batteries
α-MnO2 is a potential positive electrode material for aqueous zinc-ion batteries, but its electrochemical performance of zinc storage requires further improvement. In this paper, potassium ion-doped manganese dioxide nanoscrolls (K![]() MnO2) with oxygen vacancy were synthesized by a one-step hydrothermal method. It was observed that the electrochemical specific capacity was 250.9 mAh/g at a current density of 0.2 C, which was better than the existing commercial α-MnO2. At a high current of 1 C, these batteries demonstrate improved cycle stability. Synchrotron radiation and other experiments as well as DFT theoretical calculations provided additional evidence that K doping was efficient in regulating the metal bond type and the mean charge regulation of covalent bonds with oxygen atoms in MnO2. When Mn
MnO2) with oxygen vacancy were synthesized by a one-step hydrothermal method. It was observed that the electrochemical specific capacity was 250.9 mAh/g at a current density of 0.2 C, which was better than the existing commercial α-MnO2. At a high current of 1 C, these batteries demonstrate improved cycle stability. Synchrotron radiation and other experiments as well as DFT theoretical calculations provided additional evidence that K doping was efficient in regulating the metal bond type and the mean charge regulation of covalent bonds with oxygen atoms in MnO2. When Mn![]() O and Mn
O and Mn![]() K bonds are present, K
K bonds are present, K![]() MnO2 showed outstanding adsorption of Zn2+ and further enhanced the Zn2+ embedding process. Simultaneously, oxygen defects caused by doping boosted the development of the nanoscroll structure, leading to an increase in active sites available for electrochemical reactions and subsequently enhancing the electrical conductivity of α-MnO2. This study exhibits the potential of optimizing materials based on manganese with the introduction of a potassium doping strategy, resulting in improved performance for aquatic zinc-ion batteries, and presents novel perspectives for related research.
MnO2 showed outstanding adsorption of Zn2+ and further enhanced the Zn2+ embedding process. Simultaneously, oxygen defects caused by doping boosted the development of the nanoscroll structure, leading to an increase in active sites available for electrochemical reactions and subsequently enhancing the electrical conductivity of α-MnO2. This study exhibits the potential of optimizing materials based on manganese with the introduction of a potassium doping strategy, resulting in improved performance for aquatic zinc-ion batteries, and presents novel perspectives for related research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


