二氧化锡/氧化铝催化剂催化二羟基丙酮转化为乳酸甲酯
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究人员使用 xSnO2/Al2O3 支持的催化剂在流动条件下将甲醇中的二羟基丙酮溶液转化为乳酸甲酯,并通过 XRD、低温氮(吸附)解吸分析和紫外可见光谱对催化剂进行了表征。研究发现,在二羟基丙酮向乳酸甲酯的选择性转化过程中,含二氧化锡催化剂表面的路易斯酸位点和布氏酸位点起着至关重要的作用。在 160°C、1.0 兆帕、进料速率为 4 毫摩尔 C3H6O3/(gcat-h)的条件下,5%SnO2/Al2O3 催化剂生成乳酸甲酯的选择性达到 90%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
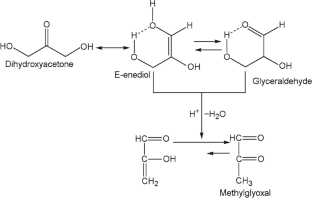
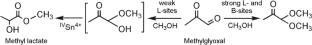
Catalytic Conversion of Dihydroxyacetone to Methyl Lactate Over SnO2/Al2O3 Catalysts
The conversion of dihydroxyacetone solution in methanol to methyl lactate has been studied in flow regime using xSnO2/Al2O3-supported catalysts that have been characterized by XRD, low-temperature nitrogen (ad)desorption analysis, and UV-Vis spectroscopy. It is found that Lewis and Brønsted acid sites of the surface of SnO2-containing catalysts play a crucial role in the selective conversion of dihydroxyacetone to methyl lactate. The formation of methyl lactate with a selectivity of 90% is achieved on 5%SnO2/Al2O3 catalyst at 160°C, 1.0 MPa, and under feed rate of 4 mmol C3H6O3/(gcat·h).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


