斯蒂尔病的最新进展和不断演变的概念
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
斯蒂尔病是一种罕见的炎症综合征,包括全身性幼年特发性关节炎和成人型斯蒂尔病,这两种疾病都可能出现危及生命的并发症,包括巨噬细胞活化综合征(MAS),这是一种继发性嗜血细胞淋巴组织细胞增多症。对斯蒂尔病的遗传学研究涉及 HLA 和非 HLA 易感基因,表明适应性免疫细胞介导的免疫参与其中。与此同时,表型证据表明该病涉及自身炎症过程。还有证据表明,I 型干扰素特征、雷帕霉素复合体 1 信号的机制靶点和铁蛋白与 Still's 病和 MAS 的发病机制有关。与斯蒂尔病相关的病理实体包括可能与生物 DMARDs 和 MAS 的发生相关的肺部疾病。从历史上看,Still's 病的病程有单相、复发性和持续性之分。新近提出的斯蒂尔病替代群可根据免疫细胞图谱更好地剖析临床异质性,而免疫细胞图谱可代表不同的内型或疾病活动阶段。在治疗方面,有关 IL-1 和 IL-6 拮抗剂以及 Janus 激酶抑制剂的数据表明,早期用药对斯蒂尔病非常重要。此外,有证据表明,IFNγ拮抗剂可用于治疗罹患MAS的患者。尽管取得了这些进展,但仍有未满足的需求,这些需求可作为设计未来研究的基础,从而改善疾病管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
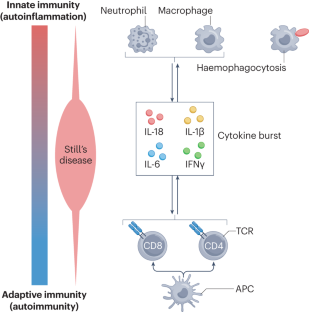
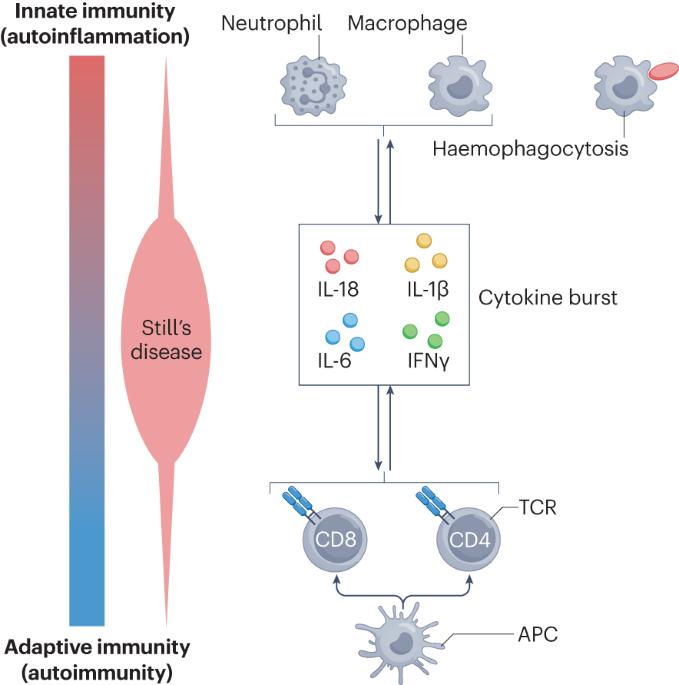
Recent advances and evolving concepts in Still’s disease
Still’s disease is a rare inflammatory syndrome that encompasses systemic juvenile idiopathic arthritis and adult-onset Still’s disease, both of which can exhibit life-threatening complications, including macrophage activation syndrome (MAS), a secondary form of haemophagocytic lymphohistiocytosis. Genetic insights into Still’s disease involve both HLA and non-HLA susceptibility genes, suggesting the involvement of adaptive immune cell-mediated immunity. At the same time, phenotypic evidence indicates the involvement of autoinflammatory processes. Evidence also implicates the type I interferon signature, mechanistic target of rapamycin complex 1 signalling and ferritin in the pathogenesis of Still’s disease and MAS. Pathological entities associated with Still’s disease include lung disease that could be associated with biologic DMARDs and with the occurrence of MAS. Historically, monophasic, recurrent and persistent Still’s disease courses were recognized. Newer proposals of alternative Still’s disease clusters could enable better dissection of clinical heterogeneity on the basis of immune cell profiles that could represent diverse endotypes or phases of disease activity. Therapeutically, data on IL-1 and IL-6 antagonism and Janus kinase inhibition suggest the importance of early administration in Still’s disease. Furthermore, there is evidence that patients who develop MAS can be treated with IFNγ antagonism. Despite these developments, unmet needs remain that can form the basis for the design of future studies leading to improvement of disease management. In this Review, the authors describe shared pathophysiology of systemic juvenile idiopathic arthritis and adult-onset Still’s disease and their life-threatening complication, macrophage activation syndrome. Therapeutic developments now enable the targeting of multiple pathways in these conditions, and evidence suggests that early use of DMARDs has the potential to prevent chronic disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


