测定 pKa 值未知的二元有机碱 4,4′-三亚甲基二哌啶 (TMDP) 的 pKa1 和 pKa2 值
IF 1.4
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在 298 K 温度下,通过 pH 度量滴定法测定了 4,4′-三亚甲基二哌啶(TMDP)的酸解离常数(pKa1 和 pKa2)的负对数值,并将实验测定的 pKa 值与 ACD/Labs 和 ChemAxon 等化学生物信息学软件预测的 pKa 值进行了比较。该实验为加深对亨德森-哈塞尔巴赫方程、酸碱解离常数(Ka/Kb)和半等价点的理解提供了极好的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
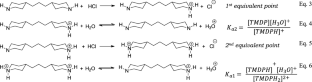
Determining the pKa1 and pKa2 Values of 4,4′-Trimethylenedipiperidine (TMDP), a Diprotic Organic Base with Unknown pKa Values
The negative logarithm of the acid dissociation constant values (pKa1 and pKa2) of 4,4′-trimethylenedipiperidine (TMDP) was determined by a pH metric titration at 298 K. The experimentally determined pKa values were compared with the predicted pKa values by chem-bioinformatics software like ACD/Labs and ChemAxon. This experiment gave an excellent opportunity to deepen engagement with the Henderson–Hasselbalch equation, acid/base dissociation constant (Ka/Kb), and half equivalence point.
Graphical Abstract
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solution Chemistry
化学-物理化学
CiteScore
2.30
自引率
0.00%
发文量
87
审稿时长
3-8 weeks
期刊介绍:
Journal of Solution Chemistry offers a forum for research on the physical chemistry of liquid solutions in such fields as physical chemistry, chemical physics, molecular biology, statistical mechanics, biochemistry, and biophysics. The emphasis is on papers in which the solvent plays a dominant rather than incidental role. Featured topics include experimental investigations of the dielectric, spectroscopic, thermodynamic, transport, or relaxation properties of both electrolytes and nonelectrolytes in liquid solutions.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


