EPAC1 可促进棕色脂肪的生长和米色脂肪的生成
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
棕色脂肪组织(BAT)是一个核心的致热器官,能增强能量消耗和心脏代谢健康。然而,专门增加产热脂肪细胞数量的调节剂仍是一个尚未满足的需求。在这里,我们发现 cAMP 结合蛋白 EPAC1 是适应性 BAT 生长的核心调节因子。在体内,选择性药理激活 EPAC1 可增加 BAT 的质量和白色脂肪的棕色化,从而增加能量消耗并减少饮食引起的肥胖。从机理上讲,EPAC1 协调了一个增殖调节因子网络,该网络专门作用于产热脂肪细胞,而不是白色脂肪细胞。我们将 EPAC1 的作用定位在 PDGFRα 阳性的前脂肪细胞上,这些细胞中 EPAC1 的缺失会阻碍 BAT 的生长并加重饮食诱导的肥胖。重要的是,EPAC1 的激活能增强人类棕色脂肪细胞和人类棕色脂肪器官组织的增殖和分化。值得注意的是,RAPGEF3(编码 EPAC1)的编码变体与体重指数呈正相关,它能抑制去甲肾上腺素诱导的棕色脂肪细胞增殖。因此,EPAC1 可能是一个有吸引力的靶点,可用于提高产热脂肪细胞的数量和能量消耗,以防治代谢性疾病。本文章由计算机程序翻译,如有差异,请以英文原文为准。

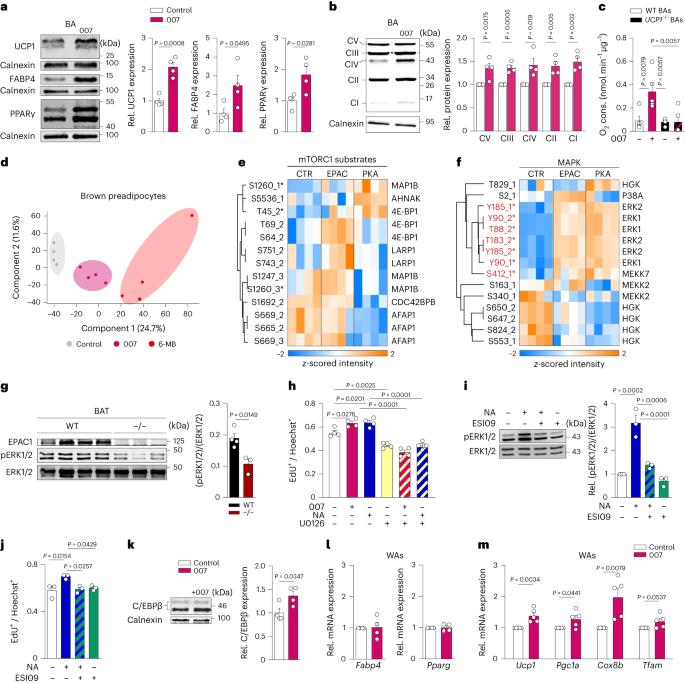
EPAC1 enhances brown fat growth and beige adipogenesis
Brown adipose tissue (BAT) is a central thermogenic organ that enhances energy expenditure and cardiometabolic health. However, regulators that specifically increase the number of thermogenic adipocytes are still an unmet need. Here, we show that the cAMP-binding protein EPAC1 is a central regulator of adaptive BAT growth. In vivo, selective pharmacological activation of EPAC1 increases BAT mass and browning of white fat, leading to higher energy expenditure and reduced diet-induced obesity. Mechanistically, EPAC1 coordinates a network of regulators for proliferation specifically in thermogenic adipocytes, but not in white adipocytes. We pinpoint the effects of EPAC1 to PDGFRα-positive preadipocytes, and the loss of EPAC1 in these cells impedes BAT growth and worsens diet-induced obesity. Importantly, EPAC1 activation enhances the proliferation and differentiation of human brown adipocytes and human brown fat organoids. Notably, a coding variant of RAPGEF3 (encoding EPAC1) that is positively correlated with body mass index abolishes noradrenaline-induced proliferation of brown adipocytes. Thus, EPAC1 might be an attractive target to enhance thermogenic adipocyte number and energy expenditure to combat metabolic diseases. Reverte-Salisa et al. show that, in preadipocytes, EPAC1 enhances brown adipose tissue growth and increases the function of thermogenic fat in obesogenic conditions. Activation of EPAC1 induces human brown adipocyte proliferation and differentiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


