新型氟化菲啶-6(5H)-酮衍生物的高效合成及其抗病毒活性的体外评估
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本研究介绍了一种在氯苯中以三氟甲磺酸为介质,从相应的 2-异氰酸基-1,1′-联苯合成氟化菲啶-6(5H)-酮的便捷而高效的方法。该反应使用现成的起始材料,在非常温和的反应条件(r. t. )下进行,可以获得具有生物前景的氟化杂环,收率良好甚至极佳。合成的化合物在 MDCK 细胞系中进行了细胞毒性体外测试,并对 A/波多黎各/8/34(H1N1)流感病毒进行了抗病毒活性测试。本文章由计算机程序翻译,如有差异,请以英文原文为准。
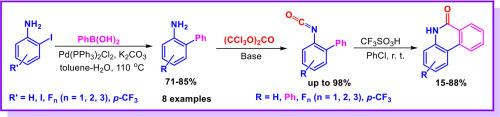
Efficient synthesis of novel fluorinated phenanthridin-6(5H)-one derivatives and in vitro evaluation of their antiviral activity
A convenient and efficient method for the synthesis of fluorinated phenanthridin-6(5H)-ones from the corresponding 2-isocyanato-1,1′-biphenyls mediated by trifluoromethanesulfonic acid in chlorobenzene is described. The reaction uses readily available starting materials, takes place under very mild reaction conditions (r. t.) and provides access to biologically promising fluorinated heterocycles in good to excellent yields. Synthesized compounds were tested in vitro for cytotoxicity in MDCK cell line and for antiviral activity against influenza virus A/Puerto Rico/8/34 (H1N1).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


