DHHC5 介导的贝克莱蛋白 1 S-棕榈酰化减少是衰老过程中自噬功能下降的基础
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
自噬是一种依赖于溶酶体的降解途径,对细胞稳态至关重要,但随着年龄的增长,自噬功能会逐渐减弱。然而,目前还不清楚衰老是如何诱导自噬功能下降的。在这里,我们展示了蛋白质 S-棕榈酰化在自噬中的作用。我们发现棕榈酰酰基酰基转移酶 DHHC5 是自噬的调控因子,它通过介导 beclin 1 的棕榈酰化,进而通过促进 beclin 1 与适配蛋白 ATG14L 和 VPS15 之间的疏水相互作用,促进含 ATG14L 的 III 类磷脂酰肌醇-3-激酶复合物 I 的形成及其脂质激酶活性。在人类和非人灵长类动物衰老的大脑中,DHHC5 的表达水平明显下降。我们的研究表明,在两种已建立的阿尔茨海默病小鼠模型中,神经元中 DHHC5 的缺乏会导致细胞蛋白质平衡的降低,从而以依赖自噬的方式加剧神经退行性变。这些发现确定了 DHHC5 介导的贝克莱蛋白 1 S-棕榈酰化减少是衰老诱导自噬衰退的潜在机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
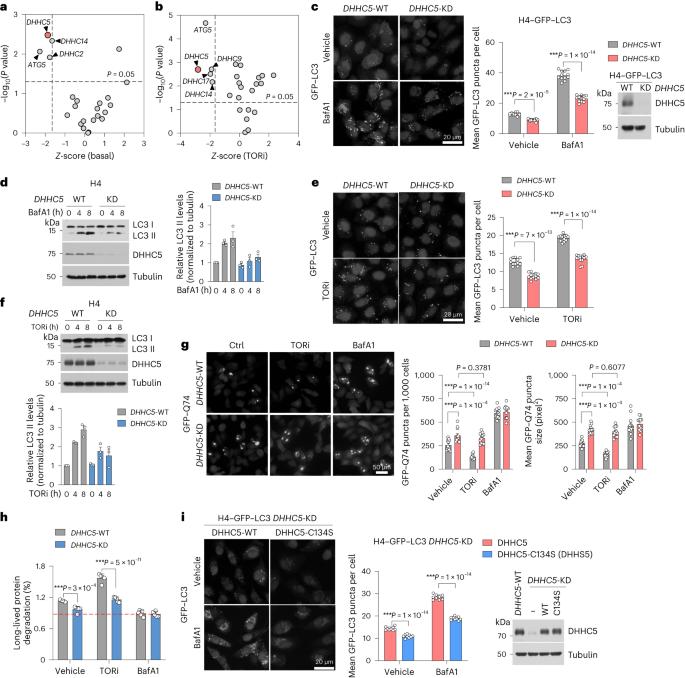
Reduction of DHHC5-mediated beclin 1 S-palmitoylation underlies autophagy decline in aging
Autophagy is a lysosome-dependent degradation pathway essential for cellular homeostasis, which decreases with age. However, it is unclear how aging induces autophagy decline. Here we show the role of protein S-palmitoylation in autophagy. We identify the palmitoyl acyltransferase DHHC5 as a regulator of autophagy by mediating the palmitoylation of beclin 1, which in turn promotes the formation of ATG14L-containing class III phosphatidylinositol-3-kinase complex I and its lipid kinase activity by promoting the hydrophobic interactions between beclin 1 and adapter proteins ATG14L and VPS15. In aging brains of human and nonhuman primate, the levels of DHHC5 exhibit a marked decrease in expression. We show that DHHC5 deficiency in neurons leads to reduced cellular protein homeostasis in two established murine models of Alzheimer’s disease, which exaggerates neurodegeneration in an autophagy-dependent manner. These findings identify reduction of DHHC5-mediated beclin 1 S-palmitoylation as an underlying mechanism by which aging induces autophagy decline. Autophagy is essential for cellular homeostasis which decreases with age. Here, the authors identify aging-induced reduction of DHHC5-mediated beclin 1 palmitoylation as an underlying mechanism by which aging induces autophagy decline in the brain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


